
Recent studies show that fumaric acid can slow or stop some fermentation processes. For example, adding 0.6 g/L of fumaric acid to white grape must effectively inhibits the growth of lactic acid bacteria and halts malolactic fermentation. NORBIDAR offers high-purity fumaric acid in food applications and other uses. The effectiveness of fumaric acid in food depends on the quantity used, as well as the specific fermentation process and the microorganisms involved. It is important for individuals to consider both the advantages and disadvantages before implementing this solution.
Key Takeaways
- Fumaric acid can slow or stop malolactic fermentation in wine. This helps keep wine fresh and tasty. The amount of fumaric acid used is very important. Using the right amount makes it work better to control fermentation. It is important to check pH and temperature during fermentation. This helps get the best results when using fumaric acid. NORBIDAR gives high-purity fumaric acid. This means it is safe and good for food and drinks. Fumaric acid helps keep food fresh. It also makes flavors better. This makes it useful in many products.
How fumaric acid affects fermentation

Chemical properties and acidity
Fumaric acid is a compound found in nature. It helps control fermentation. Its structure has carboxylic acid groups. These groups make it acidic. The acid lowers the pH in liquids. This makes it harder for some microbes to grow. The table below lists important chemical properties that help fumaric acid stop fermentation.
| Property | Description |
|---|---|
| Chemical Formula | HO2CCH=CHCO2H |
| Acidity | Fumaric acid is a weak acid, contributing to its acidity. |
| Functional Groups | Contains carboxylic acid groups, which are responsible for its acidic nature. |
| Reaction Characteristics | Undergoes bromination and acts as a dienophile, indicating reactivity. |
| Natural Occurrence | Widely found in nature, which may influence its role in fermentation. |
Fumaric acid’s acidity lets it change the pH in foods and drinks. This can slow or stop bad microbes from growing during fermentation.
Impact on yeast and bacteria
Fumaric acid changes how yeast and bacteria act in fermentation. Scientists have looked at its effect on lactic acid bacteria. These bacteria are common in dairy and wine. The table below shows what researchers found about Lactobacillus delbrueckii ssp. bulgaricus.
| Finding | Description |
|---|---|
| Metabolism of Fumaric Acid | All tested Lactobacillus delbrueckii ssp. bulgaricus strains metabolized fumaric acid into succinic acid during monoculture in milk. |
| Fermentation Time | 75% of the strains showed shorter fermentation time compared to the control when fumaric acid was added. |
| Gene Expression | In coculture with Streptococcus thermophilus, the expression of fumarate reductase was higher than in monoculture, indicating enhanced metabolic activity. |
Yeast and bacteria do not react the same way to fumaric acid. Some bacteria use fumaric acid as food. They turn it into other things. Adding fumaric acid can make fermentation faster and change how bacteria act. This helps people control how fast fermentation happens and what the result is.
Inhibition of malolactic fermentation
Malolactic fermentation changes how wine tastes and lasts. Fumaric acid can slow or stop this process. It does this by making the environment tough for lactic acid bacteria. Studies show that adding fumaric acid to wine can stop malolactic fermentation. This keeps wine stable. The table below shows what scientists found.
| Study Title | Findings |
|---|---|
| The Effect of Fumaric Acid on Malo-Lactic Fermentation | Additions of fumaric acid greater than 3 lb per 1000 gallons retarded malo-lactic fermentation in Malbec and Pinot noir wines. |
| Use of Fumaric Acid to Inhibit Malolactic Fermentation in Bottled Rioja Wines | Fumaric acid is an additive that can be used for wine acidification and to inhibit malolactic fermentation. |
Scientists have measured how much fumaric acid is needed to stop malolactic fermentation. Here are some important facts from these studies:
- Fumaric acid concentrations used: 300-900 mg/L.
- Effect on MLF: Inhibition of malolactic fermentation in red wines.
- pH reduction: Decreased by 0.2 units or more depending on buffer capacity.
- Specific observation: Application of 600 mg/L of fumaric acid halted MLF for over 50 days.
- Microbial impact: Cells of strain alpha (Oenococcus oeni) were undetected in specific media.
Fumaric acid works by lowering the pH. This makes it hard for lactic acid bacteria to live. This helps winemakers and food makers control fermentation and stop unwanted changes in their products.
Factors that affect how well fumaric acid works
Concentration and dosage
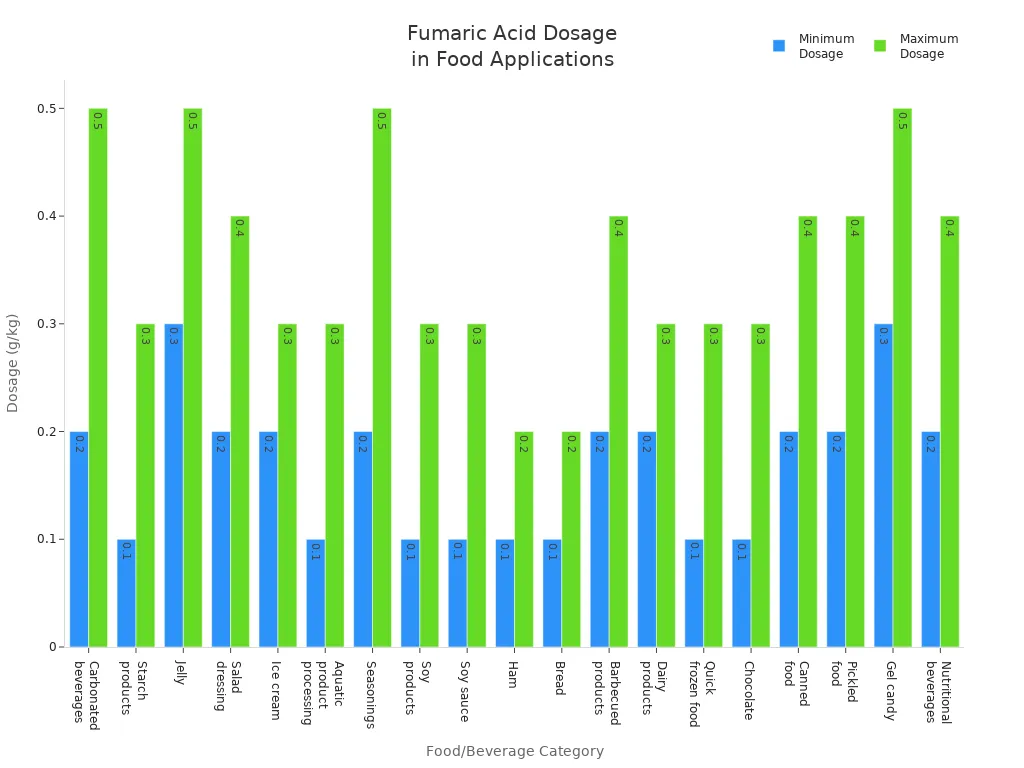
How much fumaric acid you add matters. More fumaric acid can stop more microbes. But the best amount depends on the food or drink. The table below shows the most you should use for each food.
| Food Name | Maximum Usage (g/kg) |
|---|---|
| Carbonated beverages | 0.2-0.5 |
| Starch products | 0.1-0.3 |
| Jelly | 0.3-0.5 |
| Salad dressing | 0.2-0.4 |
| Ice cream | 0.2-0.3 |
| Aquatic product processing | 0.1-0.3 |
| Seasonings | 0.2-0.5 |
| Soy products | 0.1-0.3 |
| Soy sauce | 0.1-0.3 |
| Ham | 0.1-0.2 |
| Bread | 0.1-0.2 |
| Barbecued products | 0.2-0.4 |
| Dairy products | 0.2-0.3 |
| Quick frozen food | 0.1-0.3 |
| Chocolate | 0.1-0.3 |
| Canned food | 0.2-0.4 |
| Pickled food | 0.2-0.4 |
| Gel candy | 0.3-0.5 |
| Nutritional beverages | 0.2-0.4 |
In animal feed, scientists learned fumaric acid changes fermentation in the rumen. It can lower methane and raise propionate. This happens more when animals eat less forage.
Role of pH and environment
The pH level is important for fumaric acid. Lower pH makes it harder for lactic acid bacteria to grow. When pH goes down, fumaric acid works better to stop fermentation. During fermentation, fumaric acid can get used up. So, starting with more can help slow or stop some bacteria.
Other things in the environment matter too. The table below lists two big ones:
| Environmental Factor | Impact on Fumaric Acid Production |
|---|---|
| Temperature | Affects metabolic rates and fermentation efficiency |
| Oxygen Levels | Influences the growth of microorganisms involved in fermentation |
Tip: Keeping the right temperature and oxygen helps fumaric acid work well during fermentation.
Type of fermentation process
The kind of fermentation changes how fumaric acid works. In alcoholic fermentation, yeast makes alcohol from sugar. In lactic acid fermentation, bacteria make lactic acid. The table below shows what happens to malolactic fermentation (MLF) in grape must:
| Condition | FA Addition | MLF Result | Observations |
|---|---|---|---|
| Grape Must 1 | 0 g/L | MLF occurred | Produced a lactic bite |
| Grape Must 2 | 0.6 g/L | MLF did not occur | FA decreased to 0.087 g/L during AF |
Practical uses of fumaric acid in fermentation

Controlling malolactic fermentation in wine
Winemakers use fumaric acid to help control MLF in wine. This keeps wine fresh and stable. The OIV says you can use up to 600 mg/L. Fumaric acid stops lactic acid bacteria. It keeps malic acid in the wine. It also lowers the pH by 0.05 to 0.1 units. This helps keep the wine’s taste good. Many winemakers in warm places use fumaric acid. It helps their wines stay crisp and stops unwanted changes.
| Evidence Description | Findings |
|---|---|
| Fumaric acid concentration | 300-900 mg/L stops MLF in red wines |
| pH reduction | Lowers pH by 0.2 units or more |
| Effect on MLF | 600 mg/L stops MLF for more than 50 days |
| Detection in tastings | Not found at 300-600 mg/L (p < .05) |
| Impact on SO2 levels | Lets winemakers use less SO2 and keep wine stable |
Applications in food and beverage production
Fumaric acid is used in more than wine. Food makers add it to many products. It gives candies a strong sour taste that lasts. It helps balance fruit flavors in drinks and jellies. Fumaric acid makes chewy candies feel better and less sticky. It does not soak up water easily. This helps snacks and candies stay fresh longer. Its strong acidity means you need less of it. This helps companies save money.
| Benefit | Description |
|---|---|
| Strong and long-lasting sour flavor | Makes candies taste better |
| Enhance and balance fruit flavors | Makes fruit flavors taste fresher |
| Improve texture and gelling stability | Helps chewy and gel candies keep their shape |
| Prevent moisture absorption and clumping | Keeps candy good while stored |
| Extend shelf life | Slows spoilage and keeps food fresh |
| Reduce production costs | Needs less for the same sour taste |
Tip: Fumaric acid helps food and drinks stay fresh and taste good longer.
NORBIDAR fumaric acid for quality and safety
NORBIDAR is a trusted company for pure fumaric acid. They use new technology to make sure every batch is high quality. NORBIDAR’s fumaric acid is safe for food, medicine, and industry. Each batch is tested for purity and strength. Customers can trust it for fermentation and other uses.
| Quality Control Measure | Description |
|---|---|
| Purity Assurance | NORBIDAR gives very pure fumaric acid for many uses |
| Safety Compliance | Meets safety rules for food, medicine, and industry |
| Advanced Technology | Uses new tools to keep the product pure and strong |
| Batch Consistency | Makes sure every batch is safe and pure for all uses |
Note: NORBIDAR’s focus on quality and safety makes it a great choice for people who need good fumaric acid for fermentation.
Fumaric acid vs. other fermentation inhibitors
Comparison with sorbic and benzoic acids
Many companies use acids to control fermentation. Sorbic acid and benzoic acid are used a lot. They stop yeasts and molds from growing. Fumaric acid can sometimes help fermentation happen. It also lowers ammonia in foods. The table below shows what each acid does to fermentation and ammonia:
| Acid Type | Effect on Fermentation | Effect on Ammonia Production |
|---|---|---|
| Fumaric Acid | Enhances | Reduces |
| Sorbic Acid | Inhibits | Reduces |
| Benzoic Acid | Inhibits | Reduces |
Each acid has a different chemical structure. Some have more carboxy groups or double bonds. These differences make it hard to know which acid is best every time.
Another table lists the good and bad sides of each acid:
| Acid Type | Advantages | Disadvantages |
|---|---|---|
| Fumaric Acid | Generally Regarded as Safe (GRAS) | Not much info about resistance to microbes |
| Sorbic Acid | Stops yeasts and molds well | Some microbes can resist it; can break down badly |
| Benzoic Acid | Stops yeasts and molds well | Some microbes can resist it; can break down badly |
Note: Food makers should pick the acid that is safe and fits their product.
Effectiveness versus sulfur dioxide
Sulfur dioxide is used a lot in wine making. It stops bad microbes and keeps wine fresh. Some people like acids such as fumaric acid better. These acids do not change the smell or taste much. Sulfur dioxide can cause allergies for some people. Acids are safer for those who are sensitive.
Pros and cons of fumaric acid
Fumaric acid helps keep food and drinks safe. It stops spoilage bacteria like Listeria. It keeps the right acidity in wine and grape musts. It also helps wine stay fresh by slowing fermentation. But fumaric acid does not dissolve as well as other acids. This can make it harder to use in some foods.
| Pros | Cons |
|---|---|
| Stops microbes and keeps food fresh | Does not dissolve as well as other acids |
| Works against spoilage bacteria | Harder to use in some foods |
| Keeps acidity in wine and grape musts | |
| Slows fermentation to keep wine fresh |
Tip: Producers should try different acids to see which one works best for their food or drink.
Key considerations before using fumaric acid
Assessing fermentation goals
Before you start fermentation, you should know your goals. You must decide if you want to stop, slow down, or control fermentation. Each goal needs a different method. The ingredients and additives you pick can change the final food or drink. You should think about what the microbes need and what kind of food or drink you are making. The table below lists important things to think about:
| Factor | Details |
|---|---|
| Physical and Nutritional Needs | The ratio of glucose to nitrogen is critical for high yields; an optimal C:N ratio is 200:1. |
| Neutralizing Agents | Continuous pH neutralization is necessary; CaCO3 is preferred but can cause viscosity issues. |
| Trace Metal Concentrations | Optimal concentrations for Mg++, Zn++, and Fe++ are 500 ppm, 4 ppm, and 100 ppb, respectively. |
Tip: If you set the right conditions, you can get the results you want from fermentation.
Monitoring and adjusting usage
You need to check things often during fermentation. You should look at pH, temperature, and nutrients. Sometimes you have to change how much additive you use. If the pH gets too low, some microbes might stop working. If the temperature changes, fermentation can go faster or slower. Checking these things helps you keep everything working well and stops problems.
- Use easy tools to check pH and temperature.
- Write down changes every day to help you control things.
- Change the ingredients if things are not going as planned.
Expert tips and best practices
Experts say using things like glucose can save money. Using submerged fermentation systems can help you get more product and make things work better. In the future, metabolic engineering could make it easier to ferment at low pH. The table below shows some expert ideas:
| Recommendation | Description |
|---|---|
| Use of renewable resources | Fermentation processes utilize glucose, which is three times cheaper than maleic anhydride used in chemical processes. |
| Submerged fermentation systems | These systems, when coupled with product recovery techniques, have achieved economically attractive yields and productivities. |
| Metabolic engineering | Future improvements in fumaric acid production may involve metabolic engineering approaches to facilitate low pH fermentations. |
Note: If you follow these best practices, you can get better results when using fumaric acid in fermentation.
Recent studies show important facts about fumaric acid in fermentation. It stops malolactic fermentation. This helps wine keep its sour taste and stay fresh. Wines with fumaric acid have lower pH. They also smell better. Using the right amount is important. Good process control gives steady results.
| Benefit | Description |
|---|---|
| High purity | NORBIDAR gives pure fumaric acid for fermentation. |
| Practical dosage | Advice helps producers get the best results. |
Producers who want good quality can use NORBIDAR fumaric acid. If you have special fermentation needs, talk to experts for help.
FAQ
What is fumaric acid used for in food and drinks?
Fumaric acid makes foods taste sour. It helps keep foods fresh. Companies put it in sodas, candies, bread, and jellies. It helps products last longer and stay good.
Can fumaric acid completely stop fermentation?
Fumaric acid can stop some fermentation. It works best for malolactic fermentation in wine. How well it works depends on how much you use. It also depends on what microbes are there.
Is fumaric acid safe for consumption?
The FDA says fumaric acid is safe for food. Producers must use the right amount to keep food safe.
How does fumaric acid compare to other acids like sorbic acid?
| Acid Type | Main Use | Strengths |
|---|---|---|
| Fumaric Acid | Food, wine, feed | Sour taste lasts longer |
| Sorbic Acid | Food preservation | Stops mold very well |
Fumaric acid gives food a sour taste that stays. Sorbic acid is good at stopping mold.
Where can producers buy high-quality fumaric acid?
NORBIDAR sells pure fumaric acid. The company uses new tools and checks quality. Producers can trust NORBIDAR for food, drinks, and other uses.




