
Fumaric acid is more stable than maleic acid. This is because their molecular structures differ. Fumaric acid has a more compact atomic arrangement, making the molecules less reactive. Studies show that fumaric acid has a lower heat of combustion and a higher melting point. These properties contribute to its stability. This is crucial in the fields of food, animal nutrition, and resin chemistry. Many industries require this stability to ensure product safety and optimal performance.For example, pharmaceutical fumaric acid, industrial fumaric acid, etc.
Key Takeaways
- Fumaric acid is more stable than maleic acid. This is because its trans configuration results in a more compact molecular arrangement, forming stronger bonds.
- Fumaric acid has a higher melting point of 287°C, while maleic acid’s melting point is only 130-139°C. This indicates that fumaric acid has a more robust structure and is therefore more stable.
- Fumaric acid is less reactive than maleic acid. This makes it ideal for food preservation and resin manufacturing. In many applications, it helps maintain product safety and extend its shelf life.
- Understanding different chemical bonds, such as intramolecular and intermolecular forces, helps us understand this. This is why fumaric acid is widely used in many industries.
Stability Comparison: Maleic Acid vs. Fumaric Acid
Cis and Trans Isomerism
Scientists use cis and trans isomers to describe molecular configurations. These configurations indicate how the parts of a molecule are arranged near the double bond. In the cis configuration, the two carboxyl groups are on the same side of the double bond. In the trans configuration, the two carboxyl groups are on opposite sides of the double bond.
- Maleic acid has a cis configuration.
- Fumaric acid has a trans configuration.
This difference in configuration alters the properties of the molecule. The cis configuration of maleic acid brings the two carboxyl groups closer together. The trans configuration of fumaric acid keeps them further apart. This small change has a significant impact on stability.
The trans configuration of fumaric acid makes it more stable. The cis configuration of maleic acid reduces its stability and increases its reactivity.
Structural Impact on Stability
The way the atoms in these acids are connected affects their stability. Studies have shown that maleic acid is almost planar. It forms strong bonds within the molecule called intramolecular hydrogen bonds. These hydrogen bonds exist between its carboxyl groups. Hydrogen bonds help maintain the molecular structure. However, the cis configuration also causes the carboxyl groups to repel each other. This crowding effect reduces the stability of maleic acid.
Fumaric acid has a trans configuration. This configuration separates the carboxyl groups from each other. It reduces the crowding effect, allowing the molecules to pack tightly together in the solid. The trans configuration itself does not form strong bonds, but it allows strong bonds to form between different molecules. This packing and bonding makes fumaric acid more stable.
Stability comparisons use thermodynamic data to explain this difference. The table below shows the enthalpy change (ΔH) and Gibbs free energy change (ΔG). Scientists use these values to measure stability.
| Source | ΔH (×10^7 J kmol⁻¹) |
|---|---|
| This research | –2.39 |
| Previous study | –2.83 |
| Literature report | –2.28 |
| Temperature (K) | ΔG (×10^7 J kmol⁻¹) |
|---|---|
| 463 | –1.43 |
| 473 | –1.41 |
| 483 | –1.40 |
| 493 | –1.36 |
These values indicate that fumaric acid has low energy and high stability. Maleic acid has high energy and low stability.
The table in the textbook shows further differences:
| Property | Maleic Acid (Cis) | Fumaric Acid (Trans) |
|---|---|---|
| Stability | Less stable | More stable |
| Solubility in Water | Soluble | Insoluble |
| Melting Point (°C) | 130 – 139 | 287 |
| Heat of Combustion (kJ/mol) | 22.7 | N/A |
The calculation model is consistent with these results. They show that the lowest energy form of maleic acid is almost planar. It forms a seven-membered ring through hydrogen bonds. Fumaric acid has several stable forms. All forms are trans configurations and have similar energies.
Maleic Acid vs. Fumaric Acid Bonding and Molecular Structure
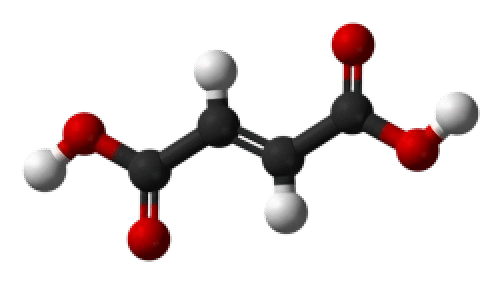
Intramolecular vs. Intermolecular Forces
The intramolecular and intermolecular forces of maleic acid and fumaric acid are different. In maleic acid, the two carboxylic acid groups are located on the same side. This allows the molecule to form hydrogen bonds with itself. This type of bond is called an intramolecular hydrogen bond. It helps to bind parts of a molecule together, but it also prevents that site from binding with other molecules. Therefore, maleic acid cannot effectively bind with its neighboring molecules, leading to reduced stability.
The carboxylic acid group is located on both sides of the molecule. This structure prevents it from forming intramolecular hydrogen bonds; both hydrogen bond sites are open. Strong intermolecular bonds, called intermolecular hydrogen bonds, can form. These hydrogen bonds help the molecule bind tightly in the solid state and also make fumaric acid more stable.
- Maleic acid uses only one hydrogen bond site to form an intramolecular hydrogen bond, which limits its ability to bind with other molecules.
- Fumaric acid has two open hydrogen bond sites, allowing it to form stronger hydrogen bonds with other molecules.
- The stronger hydrogen bonds in fumaric acid make it more stable than maleic acid, maintaining a solid state even at higher temperatures.
Steric Hindrance and Packing
The arrangement of molecules in a solid affects its properties. The cis configuration of maleic acid results in the carboxylic acid groups crowding together, a phenomenon known as steric hindrance. This prevents the molecules from packing tightly. The trans configuration of fumaric acid, on the other hand, separates the groups, resulting in a more orderly and compact molecular arrangement. This better packing gives fumaric acid a higher melting point and greater stability.
| Property | Maleic Acid (cis) | Fumaric Acid (trans) |
|---|---|---|
| Hydrogen bonding | Intramolecular | Intermolecular |
| Molecular packing | Less efficient | More efficient |
| Melting point | 130°C | 287°C |
Good packing and strong hydrogen bonds help fumaric acid maintain its solid state and stability, making it suitable for applications in many industries.
Maleic Acid vs. Fumaric Acid Physical Properties and Stability

Melting Point and Solubility
Maleic acid and fumaric acid differ in some ways. These differences help scientists understand why fumaric acid is more stable.
- Maleic acid has a lower melting point.
- Fumaric acid has a much higher melting point.
- Maleic acid is readily soluble in water.
- Fumaric acid is insoluble in water.
The table below lists their melting points and water solubility:
| Acid | Melting Point (°C) | Solubility in Water |
|---|---|---|
| Maleic Acid | 130 – 131 | Soluble |
| Fumaric Acid | 287 | Insoluble |
A lower melting point means that maleic acid melts more quickly. This indicates weaker intermolecular bonds. Fumaric acid remains solid until the temperature rises. This indicates stronger intermolecular bonds. The high melting point of fumaric acid indicates its greater stability.
Solubility also tells a story. Maleic acid is readily soluble in water. Fumaric acid is insoluble in water. Easily soluble substances generally have weaker bonds. The insolubility of fumaric acid indicates a robust structure and high stability.
Scientists use melting point and solubility to determine the strength and stability of a substance. The high melting point and low solubility of fumaric acid make it ideal for many applications.
Crystal Structure
Crystal structure alters the arrangement of molecules in a solid. Maleic acid forms relatively loose crystals. The cis configuration causes molecules to crowd and cannot align correctly. This loose arrangement results in a lower melting point and poorer stability.
Fumaric acid forms well-aligned crystals. The trans configuration allows molecules to spread out and pack tightly together. This tight packing results in a robust solid. The crystal structure of fumaric acid allows it to remain solid and stable in many situations.
The robust crystal structure gives fumaric acid a high melting point. This makes it valuable in the fields of food, animal nutrition, and resin chemistry.
Maleic Acid vs. Fumaric Acid Chemical Properties and Reactivity
Acidity and pKa Values
Scientists use the pKa value to measure how easily an acid releases hydrogen ions. The lower the pKa value, the stronger the acid. Maleic acid has pKa values of 1.92 and 6.23. Fumaric acid has pKa values of approximately 3.03 and 4.44. This means that maleic acid is a stronger acid, making it more likely to release protons.
- Maleic acid: pKa₁ = 1.92, pKa₂ = 6.23
- Fumaric acid: pKa₁ ≈ 3.03, pKa₂ ≈ 4.44
The lower pKa value of maleic acid indicates that it reacts more quickly in water. The higher pKa value of fumaric acid indicates that it retains protons for a longer period. This helps explain why fumaric acid is more stable. Its trans conformation makes its molecular structure more stable and less prone to reaction.
Scientists have chosen fumaric acid for products requiring longer shelf lives and safer storage.
Reactivity Differences
Maleic acid and fumaric acid react differently from other chemicals. Their structures affect reaction rates. The table below shows the reactions of each acid with ozone and hydroxyl radicals (OH).
| Acid Type | Reactivity with Ozone | Reactivity with OH Radicals | Induction Period in Competition with Phenol |
|---|---|---|---|
| Maleic Acid | Slower | Same rate as fumaric | Yes |
| Fumaric Acid | Faster | Same rate as maleic | No |
Fumaric acid reacts faster with ozone. Both acids react with hydroxyl radicals (OH) at the same rate. Maleic acid has an induction period with phenol, while fumaric acid does not. These differences affect how each acid functions in actual products.
The stable structure of fumaric acid makes it widely used. It is used to control the acidity of food and keep animal feed fresh. It is also used in the manufacture of polymer composites. Fumaric acid also contributes to the manufacture of batteries and specialty tablets. Its stable structure and stable reactivity make it suitable for a variety of applications.
Fumaric acid’s unique reactivity and stability make it widely used in many industries.
Fumaric Acid Stability in Industry
Food and Nutrition Applications
Fumaric acid is very important in the food and nutrition industry. Its excellent stability helps maintain food safety and freshness for extended periods. Many companies use fumaric acid to control the acidity of food and prevent spoilage. It helps balance pH levels, thereby improving the taste of food and extending shelf life. You can find it in soft drinks, jellies, and baked goods. Food-grade fumaric acid is popular due to its excellent performance in beverages, baking, and confectionery.
NORBIDAR’s fumaric acid is made from premium materials. It holds multiple certifications demonstrating its safety and reliability:
| Certification Type | Description |
|---|---|
| GMP Certification | Companies follow Good Manufacturing Practices. |
| GLP Certification | Follows Good Laboratory Practices. |
| ISO Certification | Meets world quality standards. |
| STAR-K Kosher | Needed for US food products. |
| Fumaric Acid Standards | Follows IP/BP/USP/FCC food-grade rules. |
Multiple agencies in the US, Europe, and Australia consider fumaric acid safe for food. The US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) also consider fumaric acid safe when used properly.
Fumaric acid has a robust structure, making it ideal for maintaining food safety and quality.
Resin Chemistry and Other Uses
Fumaric acid is also widely used in many factories. Its robust structure allows it to be used to produce tough and stable resins. Companies use fumaric acid to produce paper resins, alkyd resins, and unsaturated polyester resins. These resins can be used to manufacture plastics and coatings, but these products decompose over time.
| Application Type | Percentage of Annual Production | Advantages |
|---|---|---|
| Paper Resins | 35% | Safe and makes polymers harder |
| Alkyd Resins | 6% | Better than other resins in some ways |
| Unsaturated Polyester Resins | 15% | Helps make plastics that break down |
Fumaric acid helps extend the lifespan of these materials and improve their performance. The fumaric acid market is growing year by year. Experts predict that its market size will exceed US$808 million by 2035. Food-grade fumaric acid accounts for approximately 65% of total sales. The Asia-Pacific region has the largest consumption of fumaric acid, especially with the increasing demand for processed foods.
- Fumaric acid was chosen because of its safety, high strength, and wide range of applications.
- Its stable structure and certifications make it the preferred choice for many companies.
Fumaric acid is more stable than maleic acid. Its trans configuration contributes to a close molecular arrangement, forming strong bonds. Fumaric acid has a higher melting point and lower reactivity than maleic acid. The following table lists their differences:
| Property | Maleic Acid | Fumaric Acid |
|---|---|---|
| Dipole Moment | Polar | Nonpolar |
| Melting Point | Lower melting point | Higher melting point |
| Boiling Point | Boils more easily | Harder to boil |
| Solubility | Dissolves quickly in water | Does not pull water molecules close |
| Stability | Reacts quickly | Stays stable in most reactions |
NORBIDAR’s fumaric acid helps ensure food safety and enhance the strength of animal feed, and also makes resins more durable. For more information on isomer stability or industrial chemistry, please refer to the following books:
- Browning E., Buhler D. R., Reed D. J., Toxicity and Mechanisms of Industrial Solvents, 2nd ed., Elsevier, Amsterdam, 1989.
- O’Neil M. J., The Merck Index: An Encyclopedia of Chemical, Drugs and Biologicals, 14th ed., RSC, Cambridge, 2006.
- Ivanova E. P., Kostova M. A., Koumanova B. K., Asia-Pac. J. Chem. Eng. 2010, 5, 869–881.
FAQ
Why is fumaric acid more stable than maleic acid?
Fumaric acid exists in a trans configuration. This configuration helps the molecules to pack together tightly, forming strong bonds. These properties make fumaric acid more stable.
Why does fumaric acid have a higher melting point?
Fumaric acid molecules pack together tightly in a solid state, requiring higher temperatures to separate them. This is why fumaric acid has a higher melting point.
What are the industrial applications of fumaric acid?
Fumaric acid is widely used in the food and animal nutrition industries. It also helps in the manufacture of high-strength resins, keeps food fresh, and helps animals maintain health.
How does NORBIDAR ensure the quality of its fumaric acid?
NORBIDAR adheres to strict production standards. The company is GMP, GLP, and ISO certified. These measures ensure the safety and effectiveness of its products.




