Heating fumaric acid can cause different things to happen. The results depend on the temperature and the environment. At medium heat, fumaric acid turns into a gas without breaking apart. This shows it can handle heat well and does not fall apart easily. If you heat it a lot, fumaric acid can change shape and lose water. This forms maleic anhydride. Fumaric acid has a trans shape. This makes it harder for changes to happen at lower heat. Scientists in labs see that these changes happen faster as the heat goes up. The changes follow patterns that can be predicted.
Fumaric Acid: Heating Effects
Sublimation of Fumaric Acid at 200 °C Without Chemical Decomposition
When scientists heat fumaric acid to 200 °C, something special happens. The solid does not melt at this temperature. It skips melting and turns straight into a gas. This is called sublimation. No chemical decomposition happens during this process. Fumaric acid melts at a much higher temperature, about 300 °C. So, at 200 °C, it does not melt. Researchers do not see any color change or breakdown at this stage. Fumaric acid stays stable at this temperature. It does not break down early.
- At 200 °C, fumaric acid:
- Sublimes (solid to gas)
- Does not melt
- Does not decompose
- Shows no color change
Note: Maleic acid, which is similar, can change into fumaric acid when heated above 200 °C. This shows that fumaric acid is stable at 200 °C. It prefers to sublime instead of changing chemically.
Thermal Decomposition and Transformation
When the temperature goes above 200 °C, fumaric acid acts differently. Around 230 °C, it starts to break down. The main change is that it loses water molecules. It then forms maleic anhydride. This is a chemical change. The structure of fumaric acid changes because of heat.
- Key changes during thermal decomposition:
- Fumaric acid starts to break down at about 230 °C.
- The compound loses water and forms maleic anhydride.
- In open vessels, partial carbonization can occur at similar temperatures.
- At temperatures above 350 °C, toxic fumes of maleic anhydride are released.
Turning fumaric acid into maleic anhydride is important in labs and factories. Scientists use different methods to study these changes. Liquid Chromatography-Mass Spectrometry (LC-MS) is very sensitive. It can check polar or unstable compounds right away. Gas Chromatography-Mass Spectrometry (GC-MS) needs special steps but finds volatile products. High-Performance Liquid Chromatography (HPLC) is also used. It checks purity and measures how much fumaric acid is left after heating. These methods help scientists follow the changes, find products, and measure how much is made.
A study looked at how temperature affects the yield of fumaric acid and its change from maleic acid. The researchers saw that when the temperature goes from 190 °C to 220 °C, the conversion rate goes up. This means more fumaric acid is made at first. But if you heat it for too long or at higher temperatures, the yield drops. This happens because fumaric acid starts to change back to maleic acid or even to malic acid. The study showed that the most fumaric acid is made faster at higher temperatures. But the final amount goes down as the temperature gets higher. So, the process speeds up, but you may not get more in the end.
Tip: Scientists use isotope-labeled standards like ¹³C-fumaric acid. This helps them measure more accurately during these studies.
How much fumaric acid is made depends on temperature and time. Too much heat or too long a reaction can lower the yield. This is because it changes into other products. Scientists must balance these things to get the best results.
Chemical Changes When Fumaric Acid Is Heated
Isomerization Process
When fumaric acid gets hot, its molecules can move around. This is called isomerization. Fumaric acid has a trans shape. The two carboxyl groups are on opposite sides of the double bond. This shape makes the molecule strong and stable. Because it is stable, turning fumaric acid into maleic acid takes more energy. This change does not happen easily at low heat.
Scientists checked how fast this change happens at different heats. The rate goes up as the temperature gets higher. At 190 °C, the forward rate constant is 2.5 h⁻¹. At 220 °C, it jumps to 8.71 h⁻¹. The reverse reaction, where maleic acid turns back into fumaric acid, is much slower. The table below shows these numbers:
| Temperature (K) | Forward Rate Constant (h⁻¹) | Reverse Rate Constant (h⁻¹) |
|---|---|---|
| 463 | 2.5 | <0.32 |
| 473 | 5.1 | <0.32 |
| 483 | 7.2 | <0.32 |
| 493 | 8.71 | <0.32 |
Note: The forward rate gets faster with more heat, but the reverse rate stays low.
The chart below shows that the isomerization rate constant rises as the temperature goes up:
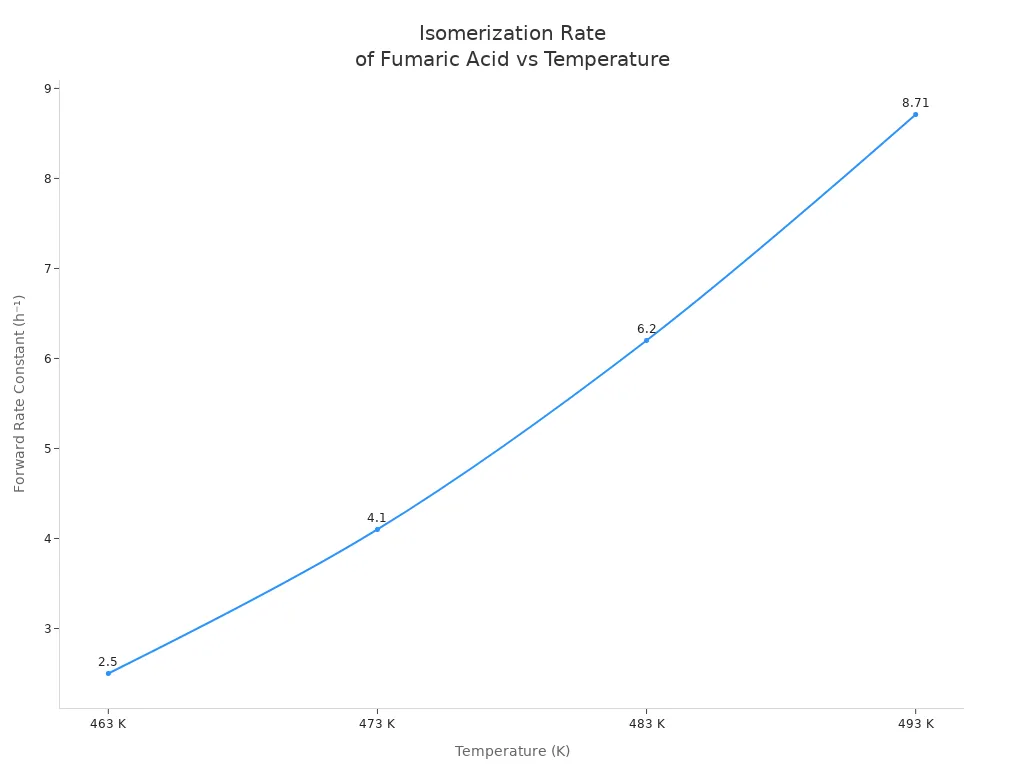
Tests in closed containers show the same pattern. If hydrochloric acid is used as a catalyst, the change happens even faster. The Arrhenius equation helps scientists guess how the rate changes with heat. As the temperature goes up, fumaric acid turns into maleic acid faster. But other reactions can also happen. This can change how much fumaric acid is left and how well the process works. The yield depends on both heat and time. Higher heat makes the change faster, but if you wait too long, you get less in the end.
Formation of Maleic Anhydride
When fumaric acid is heated above 213°C, a new change happens. The molecule loses water. This is called dehydration of fumaric acid. This step makes maleic anhydride. The reaction gets easier as the temperature goes up because entropy increases. At about 250°C, the reaction happens more on its own. Taking water away, often with phosphorus pentoxide, helps the change and gives a better yield.
In labs and factories, scientists use phosphorus pentoxide to help this reaction. This chemical soaks up water and lets the change finish. Tests show that the yield of maleic anhydride can reach up to 96% with this method. Without a dehydrating agent, the yield is lower and the change does not go as far. Fumaric acid does not lose water easily at low heat. Only when heated above 200°C does the change to maleic anhydride work well.
The trans shape of fumaric acid makes it harder to change. The molecule needs more energy to break its strong shape. This is why the change and dehydration steps need more heat than maleic acid, which has a cis shape and reacts more easily. The yield of fumaric acid and its change to maleic anhydride depend on careful control of heat and reaction steps.
Tip: For the best yield, scientists say to heat fumaric acid above 200°C and use a dehydrating agent to take away water as it forms.
Turning fumaric acid into maleic anhydride is important in many chemical industries. The process must balance heat, time, and the use of dehydrating agents to get the most product and avoid unwanted side products. The yield of fumaric acid and how well it changes are very important for these industrial processes.
Exceptions and Misconceptions
DL-Malic Acid Formation in Water
Some people think heating fumaric acid always breaks it down. This is not true. When fumaric acid is heated in water, it can turn into malic acid. This happens through a process called hydration. Hydration can happen with chemicals or with enzymes. If water and a catalyst are present and the temperature is high, fumaric acid changes into DL-malic acid. DL-malic acid is a mix of two forms. In living things, enzymes like fumarase help change fumaric acid into L-malic acid. This is part of the TCA cycle. For example, bacteria such as B. subtilis need this change to survive when stressed. The chemical way makes both D- and L- forms. The enzyme way only makes the L-form. This shows that how you do the reaction matters for what you get.
Toxic Fumes and Safety
Heating fumaric acid can be dangerous. The table below shows the main risks:
| Safety Aspect | Description |
|---|---|
| Partial Carbonization Temperature | Happens at about 446°F in open air. This makes maleic anhydride. |
| Toxic Fume Release | Maleic anhydride fumes can irritate and may come out when heating or burning fumaric acid. |
| Respiratory Risks | Breathing in fumaric acid dust can bother your lungs. |
| Skin and Eye Irritation | Touching eyes or skin for a long time can cause irritation. |
| Combustibility | Fumaric acid can burn but is not easy to light. |
| Chemical Reactivity | It reacts with bases, cyanide salts, and other chemicals. This can make flammable or toxic gases and heat. |
| Fire Hazards | Dust can explode. Use water fog to keep dust down. |
Good airflow and safety gear help lower the risk from fumes and dust. Labs and factories must watch the heating process. This helps stop the release of maleic anhydride and other dangerous byproducts.
Comparison with Maleic Acid
Fumaric acid and maleic acid look alike but have different shapes. Maleic acid has both carboxyl groups on the same side. Fumaric acid has them on opposite sides. This changes how they react:
- Maleic acid makes anhydride at lower heat because of its cis shape.
- Fumaric acid needs more heat to change because its trans shape makes it harder.
- Maleic acid forms a six-membered ring, so it changes more easily.
- Fumaric acid forms a seven-membered ring, which is harder and needs more heat.
- Both acids change by moving protons and using nucleophilic attacks, but their shapes decide how much energy is needed.
Some people think fumaric acid gives off carbon dioxide when heated, like other dicarboxylic acids. This is not true. Fumaric acid stays stable and does not do this. Its trans shape and zero net dipole moment stop decarboxylation. This makes fumaric acid different from cis isomers like maleic acid and malonic acid, which do give off carbon dioxide when heated.
Heating Fumaric Acid: Lab and Industry
Industrial Applications and Significance of Heating Fumaric Acid
Factories use maleic acid to make many products. They start with maleic anhydride, which comes from n-butane. Maleic anhydride is changed into maleic acid by adding water. Then, maleic acid turns into fumaric acid by isomerization. Fumaric acid is important in food as a flavoring and acidulant. It is also used to make unsaturated polyester resins. These resins are found in cars, boats, and building panels.
Factories do not change fumaric acid straight into maleic anhydride. Instead, these chemicals help many industrial processes. Maleic anhydride made from butane is a key step. It helps make other chemicals for farms, machines, and medicine. These steps are needed to make plastics and resins used every day.
Heating and converting these chemicals can harm the environment. Factories make fumaric acid sludge. This sludge has dangerous chemicals like naphthoquinone and maleic anhydride. If not cleaned, it can pollute soil and water. The process also releases VOCs. VOCs can cause air pollution and ground-level ozone. Factories use better catalysts and dust control to lower these problems.
Comprehensive Safety Precautions and Best Practices When Heating Fumaric Acid
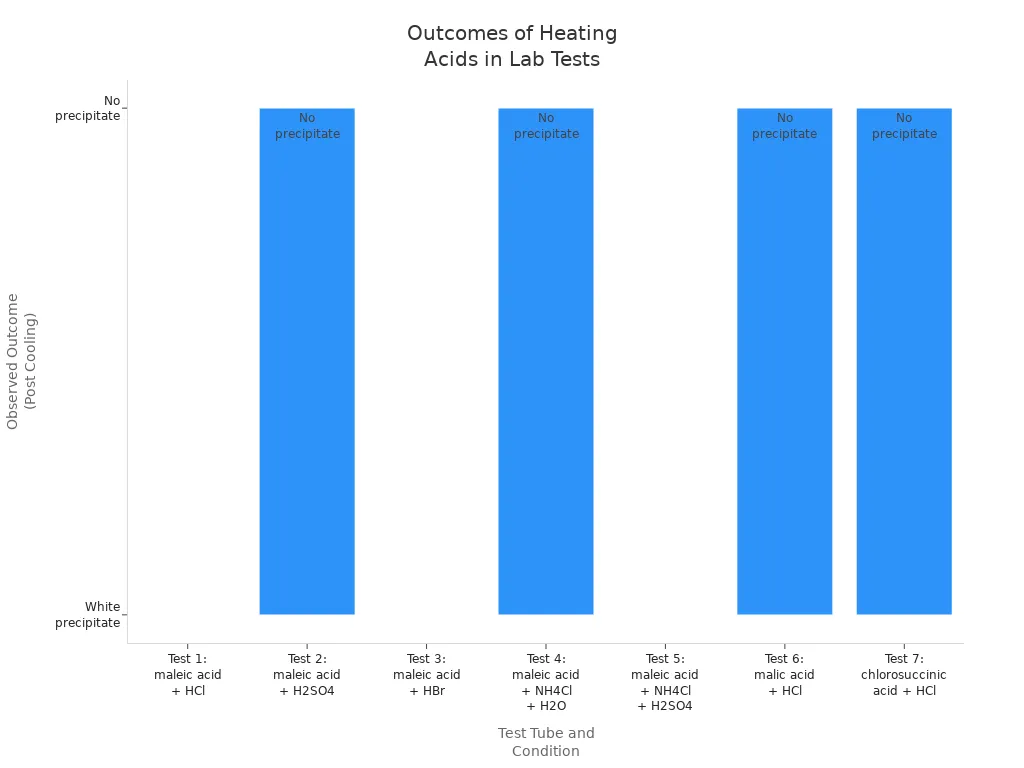
Labs follow strict rules when heating fumaric acid. This keeps the process safe and successful. The table below shows common lab tests and what happens after heating:
| Test Tube | Solid(s) Present | Solution | Heating Condition | Observed Outcome (Post Cooling) |
|---|---|---|---|---|
| 1 | 0.25 g maleic acid | 5 ml HCl | Heated | White precipitate formed |
| 2 | 0.25 g maleic acid | 5 ml H2SO4 | Heated | Clear solution (no precipitate) |
| 3 | 0.25 g maleic acid | 5 ml conc. HBr | Heated | White precipitate formed |
| 4 | 0.25 g maleic acid + 0.5 g NH4Cl | 5 ml H2O | Heated | Clear solution (no precipitate) |
| 5 | 0.25 g maleic acid + 0.5 g NH4Cl | 5 ml H2SO4 | Heated | White, cloudy precipitate formed |
| 6 | 0.25 g malic acid | 5 ml HCl | Heated | Clear solution (no precipitate) |
| 7 | 0.25 g chlorosuccinic acid | 5 ml HCl | Heated | Clear, pale yellow solution (no precipitate) |
The chart below shows which tests make a white precipitate. This means the conversion worked:
Best ways to heat fumaric acid include:
- Using closed systems to keep dust and VOCs inside.
- Wearing safety gear to protect skin and lungs.
- Watching the temperature to control the reaction and stop overheating.
- Cleaning up waste like fumaric acid sludge to protect the environment.
- Using dust collectors and good catalysts to lower emissions.
Tip: Factories and labs should always follow local safety and environmental rules when working with fumaric acid.
Heating fumaric acid gives different results depending on the heat and setting. If you use medium heat, fumaric acid turns into a gas but does not break apart. If you heat it a lot, chemical changes happen. It can turn into maleic anhydride or DL-malic acid if the right things are present. Because fumaric acid has a trans shape, it needs more heat to change. The table below shows important facts about how it reacts to heat:
| Parameter | Observation |
|---|---|
| Sublimation Temperature | ~200 °C |
| Melting Point | ~280 °C |
| Chemical Transformation | >213 °C (maleic anhydride formation) |
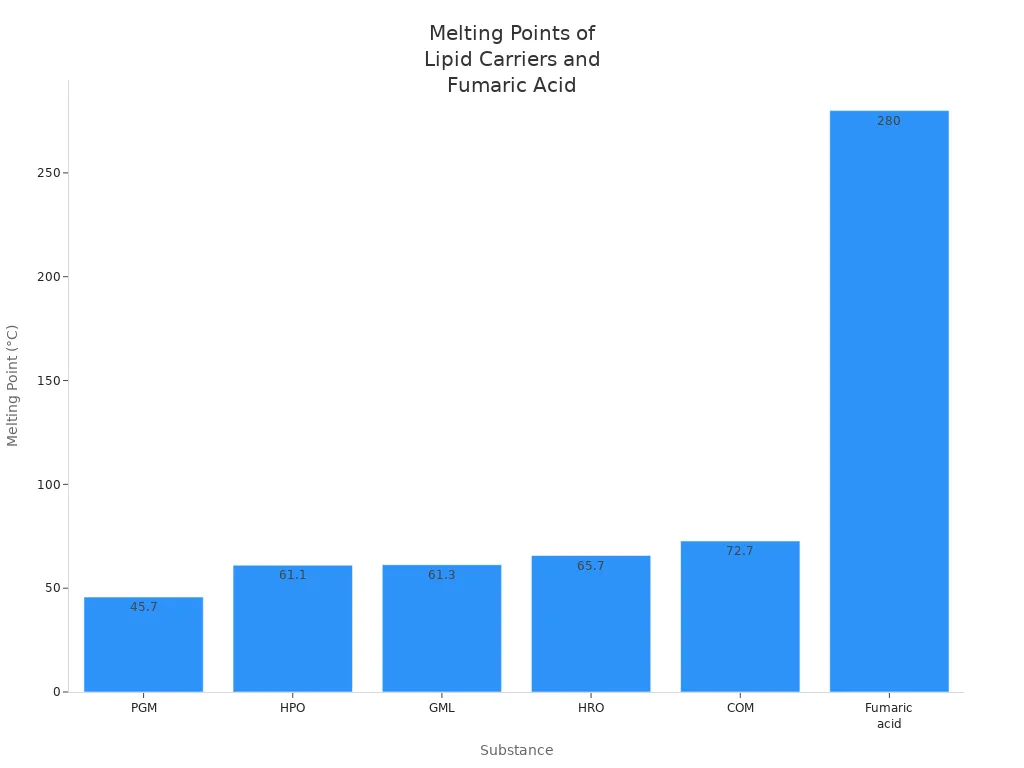
Fumaric acid can handle heat well. This lets it help make strong and tough polymers in chemistry. How hot it gets and its shape decide what happens in the reaction.
FAQ
What happens if someone accidentally inhales fumes from heated fumaric acid?
He might feel his nose, throat, or lungs get sore. Experts say to go outside for fresh air. If he still feels bad, he should see a doctor. Good airflow and safety gear help stop this problem in labs or factories.
Can heating fumaric acid cause an explosion?
Heating fumaric acid does not make explosions happen. But its dust can catch fire and be risky. Labs and factories use special machines to keep dust and fire danger low.
Is it safe to heat fumaric acid at home for experiments?
It is not safe to heat fumaric acid at home. The process can make harmful fumes and dust. Only people with training should work with this chemical. They need safety gear and special places to do it.
Does fumaric acid change color when heated?
Fumaric acid stays white at medium heat. It does not change color when it turns into a gas. At high heat, it can change in other ways. But color is not a good way to tell if it breaks down.
What should someone do if fumaric acid spills during heating?
He should put on gloves and a mask first. Use a wet cloth to clean up the powder. Open windows to let air in. Throw away the waste the right way. Do not make dust clouds while cleaning.




