
Fumaric acid is a versatile compound with varying solubility in different liquids. If you’re wondering what is fumaric acid, it’s an organic acid widely used in food, pharmaceuticals, and industrial applications. The ability of fumaric acid to dissolve in water, alcohols, and oils differs, which affects its functionality across various sectors. Below is a table showing how much fumaric acid can dissolve in common solvents at 25°C:
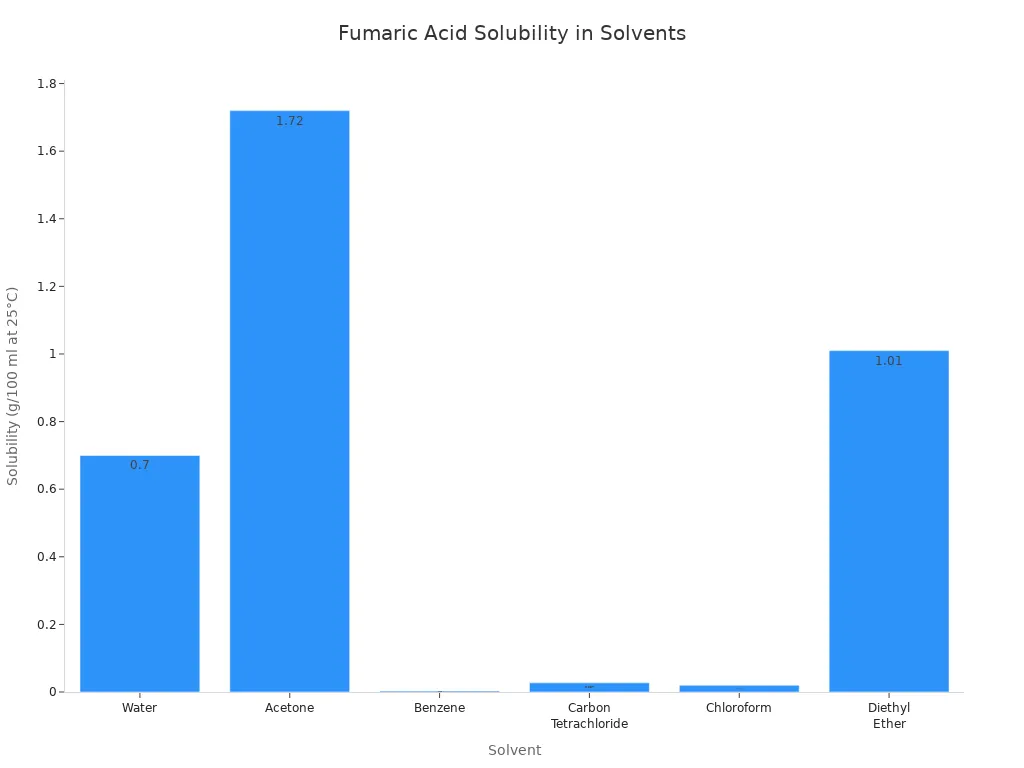
| Solvent | Solubility (g/100 ml) at 25 °C |
|---|---|
| Water | 0.70 |
| Alcohols | Soluble |
| Acetone | 1.72 |
| Benzene | 0.003 |
| Carbon Tetrachloride | 0.027 |
| Chloroform | 0.02 |
| Diethyl Ether | 1.01 |
When searching for reliable fumaric acid manufacturers, NORBIDAR stands out for delivering high-purity fumaric acid. The solubility of fumaric acid in water, alcohols, and oils plays a key role in its applications in food, pharmaceuticals, and industrial products. If you’re interested in what is fumaric acid and how it’s used, its solubility profile is essential for understanding its benefits in different industries.
Key Takeaways
- Fumaric acid dissolves best in alcohols like ethanol. This makes them good for food and medicine uses. Temperature changes how well it dissolves. Warm water helps fumaric acid dissolve better than cold water. The pH level of water also matters. It changes how fumaric acid mixes with other things. In basic water, it forms more soluble salts. Using powdered fumaric acid helps it dissolve faster. This is because powder has more surface area than granules or crystals. Fumaric acid is useful in food and animal feed. It makes food taste better and helps gut health.
Fumaric acid solubility in water

Water solubility data and limits
Fumaric acid can dissolve in water, but not much. At 25°C, about 6.3 grams can dissolve in one liter. That is the same as 0.7 grams in 100 milliliters. Scientists say this means it is “slightly soluble.” If you add fumaric acid to water, only a little bit will dissolve. The rest will stay as crystals at the bottom. This is important for people who make food and drinks. They need to measure carefully so there are no extra crystals in their products.
Temperature effects on dissolution
Temperature changes how much fumaric acid can dissolve in water. Cold water can only dissolve less than 0.1 grams in 100 milliliters. When water gets warmer, more fumaric acid can dissolve. For example:
- Cold water does not dissolve much fumaric acid.
- Warm water can dissolve more.
- Hot water can dissolve even more than cold water.
This helps people in factories and labs. They heat water to make fumaric acid dissolve faster and better.
Influence of pH on solubility
The pH of water also changes how well fumaric acid dissolves. In water that is neutral or a little acidic, not much will dissolve. If the water is more basic, the acid reacts with the base. This makes salts called fumarates, which dissolve much better in water. For example, sodium fumarate can dissolve more than fumaric acid. This is useful in food science and medicine. By changing the pH, people can control how much fumaric acid or its salts dissolve.
Tip: If you want to dissolve fumaric acid for recipes or science, use warm water and check the pH for the best results.
Fumaric acid in alcohols and solvents

Solubility in ethanol and propan-2-ol
Fumaric acid mixes much better with alcohols than with water. Ethanol and propan-2-ol are used a lot in labs and factories. Scientists check how much fumaric acid can mix in these alcohols. They find alcohols work really well. In 95% alcohol at 30°C, almost 98 grams of fumaric acid can mix in 100 grams of alcohol. This is way more than in water. People use this to make solutions for food, medicine, and chemicals.
Here is a table that shows how fumaric acid mixes in different alcohols:
| Solvent | Temperature Range (K) | Solubility of Fumaric Acid (g/100 mL) |
|---|---|---|
| Ethanol | 279 to 352 | Very high (up to 98 g/100 g at 30°C) |
| Propan-2-ol | 279 to 352 | Very high |
Note: Alcohols help mix fumaric acid fast and fully. This makes them helpful for many industries.
Comparison with acetone and other solvents
Some solvents are better than others for mixing fumaric acid. Acetone is another solvent that mixes fumaric acid well. At 25°C, acetone can mix about 1.72 grams in 100 milliliters. This is more than water but less than alcohol. Other solvents like chloroform and benzene do not mix fumaric acid well. They are almost not able to mix it.
The table below shows which solvents mix fumaric acid best:
| Solvent | Solubility (g/L) at 25 °C | Notes |
|---|---|---|
| Alcohol (95%) | 980 | Best |
| Acetone | 17.2 | Good |
| Water | 6.3 | Low |
| Chloroform | Insoluble | Very low |
| Benzene | Insoluble | Very low |
Alcohols are the best for mixing fumaric acid. Acetone is next. Water does not work as well. Chloroform and benzene do not work much at all.
Solubility in oils and ether
Fumaric acid does not mix well in oils or ether. In oils, only a tiny bit will mix. In diethyl ether, about 1 gram mixes in 100 milliliters. This is still low compared to alcohols. People cannot use oils or ether to make strong solutions of fumaric acid. For food and factories, they pick alcohols or acetone instead.
- Oils: Only a little bit mixes
- Ether: Only a little bit mixes
Tip: Use alcohols if you need to mix fumaric acid for food, medicine, or chemicals.
Factors affecting fumaric acid solubility
Temperature and environmental impact
Temperature is very important for how well fumaric acid dissolves. When things get warmer, molecules move faster and break apart easier. This makes the acid dissolve faster and in bigger amounts. At room temperature, only a little bit will dissolve. If you heat the water, a lot more can mix in. Factories change the temperature to get the best results. The environment also matters. Humidity can change how much water the acid takes in before it dissolves. Some additives, like dioctyl sodium sulfosuccinate, help the acid dissolve better. The size of the particles is important too. Small particles dissolve faster than big ones.
Tip: If you heat the liquid and use small powders, fumaric acid will dissolve better.
pH and chemical interactions
The pH level changes how fumaric acid works with other chemicals. In cold water, the acid does not dissolve much. When water is hot, the acid dissolves better. If the water is more basic, the acid reacts and makes salts called fumarates. These salts dissolve easier than the acid. The acid is hydrophobic, so it does not mix with water as well as other acids like citric or malic acid. This is good for products that need to stay dry.
- Cold water: Not much dissolves
- Hot water: More dissolves
- Basic water: Makes salts that dissolve well
- Hydrophobic nature: Good for moisture-resistant things
Physical form and concentration
The way fumaric acid looks changes how fast it dissolves. Powders have more surface area than granules or crystals. Powders dissolve the fastest. Granules and crystals take longer because they have less surface area. The crystal form helps control how fast the acid dissolves. This is important for products that need the acid to dissolve slowly. How much acid you add also matters. If you add too much, only some will dissolve and the rest will stay solid.
- Powders: Dissolve fast
- Granules: Dissolve at a medium speed
- Crystals: Dissolve slowly and in a controlled way
- High concentration: Only some will dissolve
Note: Picking the right form and amount helps get the effect you want in food, feed, and industrial products.
Practical uses of fumaric acid based on solubility
Food and beverage applications
Fumaric acid is important in food and drinks. Companies use it to make drinks taste sour. It is very strong, so they need less of it. This saves money for manufacturers. The acid lowers the pH, which keeps drinks safe. Dry drink mixes stay fresh because fumaric acid does not soak up much water. This stops clumps from forming. Sour candies and tart drinks use it for a long-lasting sour taste.
| Application Type | Description |
|---|---|
| Beverages | Adds sourness and keeps pH steady in mixes. |
| Cost-Effectiveness | Less is needed because it is very strong. |
| Superior Stability | Stops clumping in dry mixes. |
| Long-Lasting Tartness | Keeps sour flavor in candies and drinks. |
Fumaric acid helps keep food safe by lowering pH. It stops germs from growing. It can dissolve in water and alcohol, so it works in many recipes.
Animal nutrition and feed benefits
Farmers put fumaric acid in animal feed. It helps animals grow and digest food better. The acid lowers bad bacteria in the gut. This keeps animals healthy. Fumaric acid mixes well in feed, so every animal gets the same amount. Chickens, pigs, and fish get better gut health and grow faster.
- Helps animals digest food and grow
- Lowers bad bacteria in the gut
- Mixes evenly in animal feed
Industrial and personal care uses
Factories use fumaric acid in many products. In baking, hot water-soluble types release acid slowly. This helps control baking. Cold water-soluble types work fast in sauces and drinks. Fumaric acid makes plastics and coatings stronger and more heat-resistant. Skin care products use it for gentle exfoliation. It helps improve skin tone, even for sensitive skin.
| Form | Solubility | Applications | Release Rate |
|---|---|---|---|
| HWS | Dissolves in hot water | Used in baking and hot foods | Slow acid release |
| CWS | Dissolves in cold water | Used in drinks, sauces, and cold foods | Fast acid release |
- Used in plastics, coatings, and glues for strength
- Helps gently exfoliate skin in personal care items
Pharmaceutical applications
Medicine makers use fumaric acid in pills. It helps pills dissolve and stay stable. This makes medicine work better in the body. Fumaric acid helps drugs for skin problems like psoriasis. It also protects nerves and fights damage from oxidation.
NORBIDAR’s pure fumaric acid is used in food, animal feed, industry, skin care, and medicine. Its ability to dissolve makes it useful for many jobs.
Fumaric acid mixes best with water and alcohols. These are good choices for food, factories, and health products. New studies show it mixes better in urine than in water. This could help doctors and scientists in the future. Safety is very important when using fumaric acid. People should wear safety gear and follow rules for throwing it away. There are laws that help pick the right solvent to keep products safe. NORBIDAR’s fumaric acid is very pure and works well. It helps make cleaner foods and better medicines for the future.
FAQ
What is the best solvent for dissolving fumaric acid?
Alcohols like ethanol are the best at mixing with fumaric acid. Water can mix some, but not as much as alcohols. Factories use alcohols because they mix the acid fast and fully.
Can fumaric acid dissolve in cold water?
Fumaric acid does not mix well in cold water. Most of it stays as crystals at the bottom. Warm or hot water helps more of the acid mix in.
Is fumaric acid safe for food and drinks?
Yes, food-grade fumaric acid is safe if you use it the right way. It keeps food fresh and makes it taste sour. NORBIDAR sells very pure fumaric acid for safe use in food and drinks.
Does the form of fumaric acid affect how fast it dissolves?
Yes. Powders mix into liquids faster than granules or crystals. Smaller pieces have more surface area, so they mix in more quickly.




