Fumaric acid is more stable than maleic acid. Quantum chemical calculations and infrared spectroscopy both show this. Fumaric acid keeps more stable shapes. Kinetic studies show that maleic acid changes into fumaric acid. This proves fumaric acid has higher thermodynamic stability. The way the molecules are shaped and bonded is important. The atoms in fumaric acid are arranged to have less strain. This makes it more stable. This difference is important in science and industry.
Key Takeaways
- Fumaric acid is more stable than maleic acid. Its molecules have less strain. They fit tightly in crystals. This makes fumaric acid strong and heat resistant.
- Maleic acid has a cis shape. This shape causes more strain. It also makes weaker bonds between molecules. So, maleic acid melts at a lower temperature. It reacts faster too.
- Fumaric acid forms strong bonds with other molecules. This creates a stable network. It helps medicines and foods last longer.
- Maleic acid dissolves easily in water. It acts as a stronger acid at first. But it is less stable. It breaks down faster when heated or in light.
- Picking the right acid based on stability and properties is important. It helps make safer and longer-lasting products in food, medicine, and industry.
Stability Comparison Between Fumaric Acid & Maleic Acid

Fumaric Acid Stability
Fumaric acid is very stable in labs and factories. Scientists check how much energy changes when maleic acid turns into fumaric acid. This reaction gives off energy, so fumaric acid is more stable. The table below shows the energy numbers for this change:
| Source | ΔH (×10^7 J kmol⁻¹) |
|---|---|
| This research | –2.39 |
| Previous study | –2.83 |
| Literature report | –2.28 |
| Temperature (K) | ΔG (×10^7 J kmol⁻¹) |
|---|---|
| 463 | –1.43 |
| 473 | –1.41 |
| 483 | –1.40 |
| 493 | –1.36 |
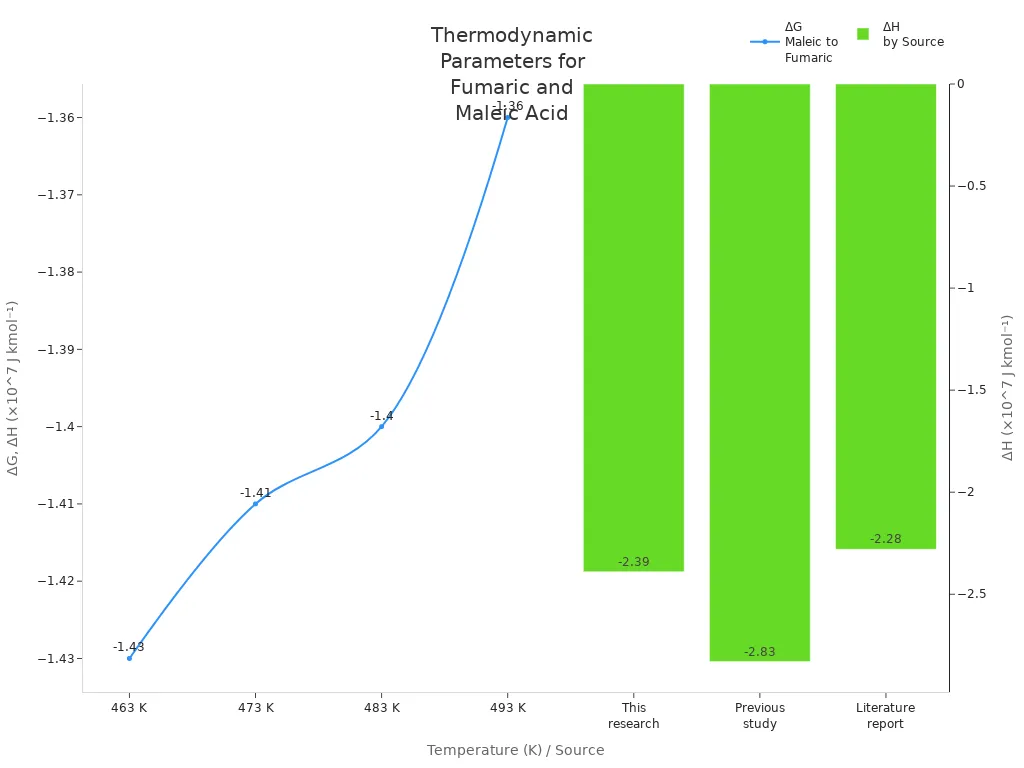
Both enthalpy and Gibbs free energy are negative. This means fumaric acid forms easily and stays stable, especially when it is cooler. Scientists see the same thing in their tests. Fumaric acid melts at 287 °C, so it does not break down easily when heated.
Fumaric acid has a crystal structure that helps it stay stable. The molecules fit together tightly. This stops them from moving too much and keeps the acid from breaking down. This helps medicines last longer and work better.
Researchers found many reasons why fumaric acid is so stable:
- It makes strong crystals that do not get ruined by water or heat.
- The acid forms salts with drugs, which keeps them safe.
- Fumaric acid keeps tablets a little acidic, stopping bad reactions.
- Tablets with fumaric acid are harder and easier to make.
- The acid helps medicines keep working well for a long time.
These things make fumaric acid a great choice for medicine and food. Chemical stability is very important in these areas.
Maleic Acid Stability
Maleic acid is not as stable as fumaric acid. Its molecule has two acid groups close together. This makes it react more easily. Maleic acid breaks down or reacts with other chemicals faster. Its heat of combustion is higher than fumaric acid, so it is less stable.
| Property | Maleic Acid | Fumaric Acid |
|---|---|---|
| Heat of Combustion | −1,355 kJ/mol | 22.7 kJ/mol lower than maleic acid |
| Melting Point | 135 °C | 287 °C |
Maleic acid melts at 135 °C, which is much lower than fumaric acid. This means maleic acid is less stable when heated. In tests, maleic acid can turn into fumaric acid. This only happens if there is acid, heat, or light. The molecule needs extra energy to twist its double bond.
Scientists also found maleic acid breaks down faster when it gets hotter. This happens even without oxygen. The acid can break down in air or nitrogen. This shows it is sensitive to heat, not just to oxygen. Because of this, maleic acid is not good for products that need to stay stable for a long time.
The cis-configuration of maleic acid makes it react more and be less stable. This means it is not the best choice when you need a stable acid.
Analysis of the Molecular Structure of Fumaric Acid and Maleic Acid
Cis Configuration in Maleic Acid
Maleic acid is the cis-form of butenedioic acid. Both carboxyl groups are on the same side. This makes the molecule act in special ways:
- The carboxyl groups are close together, so they can make h-bonds inside the molecule.
- The groups are crowded, which causes strain in the molecule.
- When heated, the cis-form can easily make a five-membered ring because the carboxyl groups are next to each other.
This shape gives maleic acid a bigger dipole moment and less symmetry. The cis shape also makes maleic acid less stable than the trans form. Scientists see that the cis shape lowers the temperature needed to make an anhydride and makes the acid react faster. The h-bonding inside the molecule changes how maleic acid acts in reactions and how it is used in factories.
The cis shape in maleic acid changes its stability, melting point, and acid strength. The way the molecule is built affects how it acts and looks.
Trans Configuration in Fumaric Acid
Fumaric acid is the trans-form of butenedioic acid. The carboxyl groups are on opposite sides of the double bond. This shape gives fumaric acid some good features:
| Property | Maleic Acid (Cis) | Fumaric Acid (Trans) |
|---|---|---|
| Geometry | Groups on same side, more strain | Groups on opposite sides, less strain |
| Symmetry | Lower | Higher |
| Stability | Lower | Higher |
| Melting Point | Lower | Higher |
The trans shape makes fumaric acid more even and less crowded. Fumaric acid cannot make h-bonds inside the molecule, but this helps it stay stable. Tests show the trans shape gives fumaric acid lower energy and more stability. This means fumaric acid does not react as much and stays strong in normal conditions.
The trans shape in fumaric acid helps it make strong crystals and have a high melting point. This is why fumaric acid is used a lot in food and medicine.
Bonding Differences Between Fumaric and Maleic Acid
Fumaric acid and maleic acid have different ways their atoms connect. This is because their shapes are not the same. Maleic acid has a cis shape. This lets it make a hydrogen bond inside itself. The carboxyl groups are close, so they can link together. This inside bond helps maleic acid stay strong after it loses a proton. That is why maleic acid is a stronger acid.
Fumaric acid has a trans shape. It cannot make a hydrogen bond inside itself. Instead, it makes bonds with other fumaric acid molecules. The carboxyl groups point away from each other. This lets fumaric acid join with its neighbors. These outside bonds make a strong network in crystals and liquids. The network helps fumaric acid stay stable and have a higher boiling point.
Tests show fumaric acid mixtures have big changes in their vibration bands. These changes mean there are many outside hydrogen bonds. Maleic acid mixtures do not show these changes. This means they have fewer outside bonds.
Computer models agree with these results. DFT calculations show fumaric acid has two places for outside hydrogen bonds. Maleic acid only has one place. The other spot is used for its inside bond. AIM analysis shows fumaric acid dimers have more electron density and Laplacian values. This means their hydrogen bonds are stronger.
| Parameter | Fumaric Acid Dimer (Cyclic) | Fumaric Acid Dimer (Open) |
|---|---|---|
| O⋅⋅⋅H Hydrogen Bond Length (Å) | 1.668 | 1.757 |
| Electron Density ρ(rc) (a.u.) | 0.0470 | 0.0354 |
| Laplacian ∇²(rc) (a.u.) | 0.1415 | 0.1273 |
Fumaric acid can make strong crystals because of its outside bonds. This helps it stay solid when heated. Maleic acid cannot make these networks. It melts and reacts faster.
Physical Properties Comparison
The Melting Points of Fumaric Acid and Maleic Acid
Melting point tells us how stable a compound is when heated. Fumaric acid melts at 287°C. Maleic acid melts much sooner, between 130°C and 139°C. Some tests show maleic acid starts melting at 121°C and finishes soon after. This big difference comes from how their molecules are shaped. Maleic acid has a cis shape. This puts the carboxyl groups on the same side. This shape lets the molecule make h-bonds inside itself. These inside bonds make the bonds between molecules weaker. So, the crystals do not pack tightly. This makes maleic acid melt at a lower temperature.
Fumaric acid has a trans shape. Its carboxyl groups are on opposite sides. This shape is more even and lets the molecules bond together better. The crystals pack tightly. The molecules need more heat to break apart. That is why fumaric acid melts at a much higher temperature. The table below shows these differences:
| Factor | Maleic Acid (cis) | Fumaric Acid (trans) |
|---|---|---|
| Molecular configuration | COOH groups on the same side (cis) | COOH groups on opposite sides (trans) |
| Crystal packing | Inefficient due to cis arrangement | Efficient, symmetrical packing |
| Intermolecular interactions | Reduced by intramolecular h-bonding | Stronger intermolecular h-bonding |
| Melting point | 130 – 139 °C | ~287 °C |
Fumaric acid has a higher melting point. This means it is more stable when heated and has stronger bonds between molecules.
The Solubility of Fumaric Acid and Maleic Acid in Water and Other Solvents
Solubility shows how structure and bonding affect how well something dissolves. Maleic acid dissolves in water very well, at 478.8 g/L (20°C). Fumaric acid only dissolves at 7 g/L (25°C). Maleic acid’s cis shape makes it more polar. This helps it mix with water. The inside h-bonds and polarity make it like water more. Fumaric acid’s trans shape is less polar. It depends more on bonds between molecules. This makes it harder to dissolve in water.
The chart below compares how well fumaric acid and malic acid dissolve in different liquids:
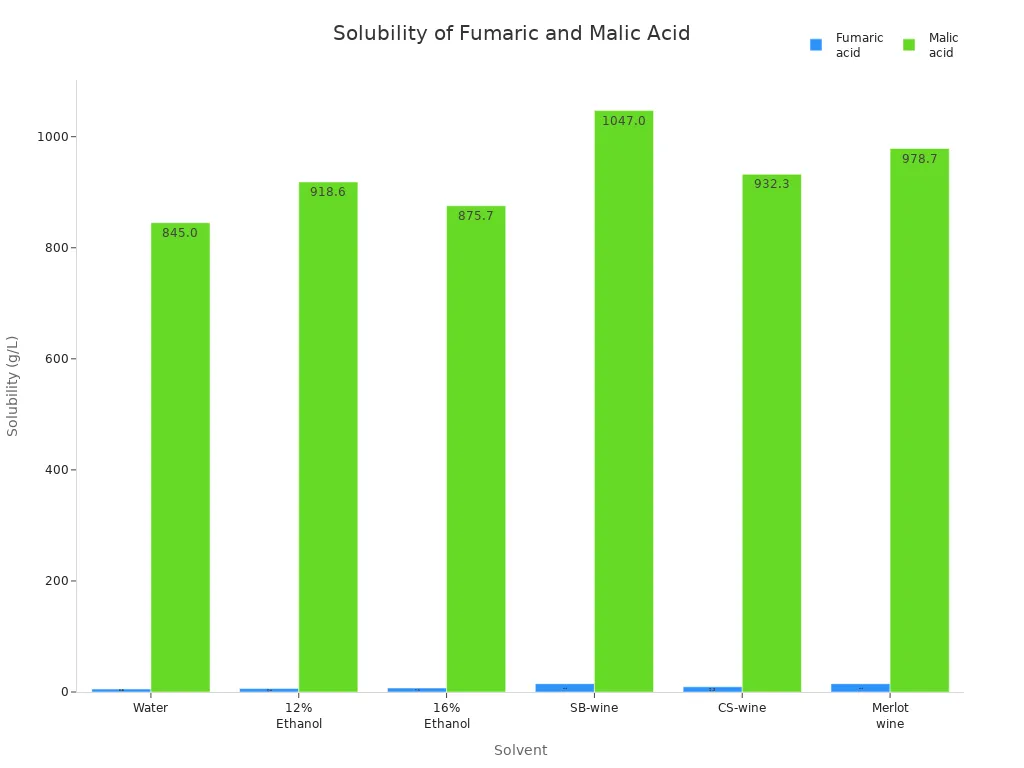
In medicine, high solubility can help drugs get into the body. Fumaric acid has higher permeability, which helps the body absorb it. People pick which acid to use based on how much they want it to dissolve, be absorbed, and stay stable when heated.
Chemical Properties Comparison
Acidity Differences Between Fumaric Acid and Maleic Acid
Acidity is about how fast a molecule gives up a proton. Maleic acid has both carboxyl groups on one side. This lets it make a hydrogen bond inside itself. That bond helps hold the negative charge after losing a proton. So, maleic acid is a stronger acid for the first step. The second proton is harder to remove. This is because the helpful bond is gone. Fumaric acid has its carboxyl groups on opposite sides. It cannot make a bond inside itself. This makes the first proton less easy to lose. But the second proton is easier to remove. The difference in acidity comes from their shapes and how they hold charges after losing protons.
Chemists pick which acid to use based on these acidity differences. The special acid strengths change how each acts in water and other liquids.
Comparative Reactivity of Fumaric Acid and Maleic Acid
These acids react differently because of their shapes. Maleic acid reacts faster in some cases. Its carboxyl groups are close together. It can make an anhydride at lower temperatures. The groups attack each other, make a ring, and lose water. Fumaric acid needs more heat for this reaction. Its carboxyl groups are farther apart. The molecule must use more energy to react.
Studies show fumaric acid breaks down faster than maleic acid sometimes. When it meets ozone or plasma, fumaric acid reacts quicker. This is true in acidic places. These different breakdown speeds show how their shapes change their reactions.
- Maleic acid makes an anhydride at lower heat because of its cis shape.
- Fumaric acid needs more energy for the same reaction because its groups are farther apart.
- Both acids can make the same product, but they need different paths and energy.
These reactivity differences change how they are used in factories. Maleic acid’s fast reactions help with quick changes. Fumaric acid’s strong stability is better for things that need to last or handle heat.
Practical Applications and Implications
Fumaric Acid and Maleic Acid in Industrial Applications
Factories need stable chemicals to make good products. Fumaric acid is made by changing maleic acid. This change is called isomerization. Workers use catalysts like phosphoric acid or enzymes called maleate cis–trans isomerase. Sometimes, they use heat-treated cells to help the process work better. Calcium ions can make the enzyme stronger and help it last longer. Companies pick different catalysts and reaction settings to get pure fumaric acid. For example, they heat maleic acid with phosphoric acid to make food-grade fumaric acid. Some methods use strong acids and pressure to make the change faster. These steps show how stability and isomerization are important for making lots of fumaric acid.
Tip: Picking the right catalyst and reaction settings helps make safe and high-quality products.
Applications of Fumaric Acid and Maleic Acid in the Food and Pharmaceutical
Food and medicine makers want chemicals that stay stable. Fumaric acid keeps drinks and baked goods fresh by controlling acidity. It does not break down when heated or wet, so foods last longer. Dimethyl fumarate, which comes from fumaric acid, helps treat diseases like multiple sclerosis and psoriasis. The trans-configuration gives fumaric acid a high melting point and strong stability. This helps products work well and last longer. In powdered foods, fumaric acid stops clumping and keeps things fresh. Groups like the FDA and EFSA say fumaric acid is safe for food. Maleic acid is mostly used to make fumaric acid because it is not as stable.
- Fumaric acid is found in:
- Baked goods
- Drinks
- Medicines
- Supplements
- Maleic acid helps make fumaric acid by controlled isomerization.
Note: Stable chemicals help food and medicine stay safe and work well for a long time.




