Fumaric Acid
Fumaric acid, or trans-butenedioic acid, is a strong acid found in plants and fruits. Made by isomerizing maleic acid, it is a white, stable crystal with a fruity smell. Its unique properties make it useful in food, drinks, polymers, and resins, boosting innovation and economic growth across many industries.
CAS
110-17-8
Chemical Formula
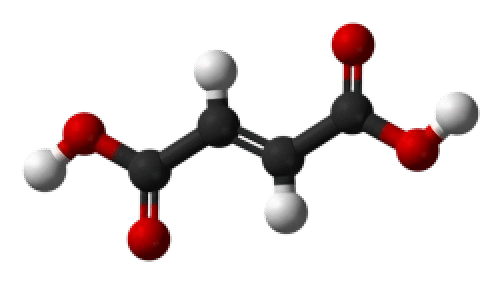
HO2CCH=CHCO2H
Molecular Weight
116.07 g/mol
Physical & Chemical Properties of Fumaric Acid
Structural Characteristics
Fumaric acid is an organic compound known as a dicarboxylic acid with the chemical formula C4H4O4. It features two carboxyl groups (-COOH) positioned across a carbon-carbon double bond in a trans configuration. This trans arrangement gives fumaric acid a more stable and less reactive structure compared to its cis-isomer, maleic acid. The molecule's planar structure and double bond position contribute to its performance in food, pharmaceutical, and industrial applications.

Acidic Behavior
As a weak organic acid, fumaric acid demonstrates predictable and controlled acidic behavior. It has two carboxyl groups that release hydrogen ions at different pKa levels. It exhibits acidic dissociation constants (pKa) around 3.03 for the first proton and approximately 4.5 for the second. This dual dissociation enables fumaric acid to act as a reliable acidulant in food and beverages. It ensures consistent pH adjustment, enhances flavor, and provides microbial stability.

Thermal and Chemical Stability
Fumaric acid shows excellent thermal stability, with a melting point of about 287°C (549°F). It remains effective under high processing temperatures and resists degradation during long-term storage. It starts to sublimate at temperatures above 200°C and decomposes rather than boiling, making it suitable for high-temperature industrial processes. Its chemical stability ensures that it does not easily react with other food or feed components, making it suitable for a wide range of uses.
Solubility and Hydrophobicity
Fumaric acid is sparingly soluble in water, with a solubility of about 6.3 g/L at 25°C, but it dissolves well in ethanol, ether, and concentrated sulfuric acid. Its moderate hydrophobicity is due to the non-polar carbon backbone despite the presence of polar carboxyl groups. This property helps it maintain low moisture absorption, which benefits its use in dry food products and industrial formulations. NORBIDAR provides both hot water soluble (HWS) and cold water soluble (CWS) forms to meet specific customer needs.
How is Fumaric Acid Produced
Fumaric acid occurs naturally in several plants and fungi. However, natural extraction is not sufficient for large-scale commercial demand, so industrial production methods are required.
Commercial fumaric acid is mainly produced through chemical synthesis and biotechnological processes. Chemical synthesis involves isomerization of maleic acid. Fermentation methods use microorganisms to convert renewable feedstocks into fumaric acid. Bioconversion techniques are increasingly adopted for their eco-friendly and sustainable nature. At NORBIDAR, we apply modern production technologies that combine efficiency, safety, and quality control.
Natural Sources of Fumaric Acid
Fumaric acid occurs naturally in certain plants and fungi. It can be found in Fumaria officinalis (common fumitory), Bolete mushrooms, Iceland moss, and various types of Lichen. These sources produce fumaric acid as part of their metabolic processes, contributing to the natural flavor and preservation qualities when used in food or herbal medicines.

Industrial Methods of Production
- Chemical Synthesis: The most common industrial method is the catalytic isomerization of maleic acid, which itself is derived from maleic anhydride. Maleic anhydride is produced by the catalytic oxidation of hydrocarbons like benzene or butane. The isomerization process converts maleic acid to fumaric acid in aqueous solutions at low pH, from which fumaric acid precipitates out.
- Fermentation: Biotechnological production using certain fungi, such as Rhizopus oryzae and Aspergillus niger, has emerged as an eco-friendly method. These microorganisms ferment renewable carbon sources like glucose or agricultural waste under controlled conditions to produce fumaric acid naturally.
- Biobased Fumaric Acid: Fumaric acid can also be produced by bioconversion using genetically modified microorganisms. This involves microbial or enzymatic conversion pathways that transform various precursors into fumaric acid through metabolic processes. For example, genetically engineered Escherichia coli (E. coli) strains have been developed to produce fumaric acid from glucose or other sugars.
Sourcing from NORBIDAR
Fumaric Acid Manufacturer
NORBIDAR is committed to supplying high-quality fumaric acid and tailored solutions that meet diverse industrial needs. Our strict quality control, advanced production capabilities, and customer-focused service ensure consistent product performance and reliability for every application. Choose NORBIDAR for fumaric acid solutions that drive innovation and success.
HWS Fumaric Acid
NORBIDAR offers Hot Water Soluble (HWS) fumaric acid designed for industries where quick dissolution at elevated temperatures is critical. This form is ideal for baked goods, beverages, and industrial resins. Our HWS fumaric acid maintains high purity and consistent quality, meeting strict industry standards for food, pharmaceuticals, and personal care formulations.
CWS Fumaric Acid
Cold Water Soluble (CWS) fumaric acid is another specialized solution from NORBIDAR. It is processed to achieve rapid solubility even at low temperatures. This makes it highly effective for juice formulations, instant drink powders, and pharmaceutical suspensions. With NORBIDAR CWS fumaric acid, customers gain efficiency and product quality without additional processing steps.
NORBIDAR Fumaric acid
Specifications
| Assay (%) | 99.5-100.5% |
| Maleic acid by HPLC (%) | Max 0.1 |
| Loss on drying (%) | Max 0.5 |
| Residue on ignition (%) | Max 0.1 |
| Melting Range | 286 – 302 °C |
| Arsenic | Max 3 mg/kg |
| Lead | Max 2 mg/kg |
| Mercury | Max 1 mg/kg |
Hazard Symbols

Applications of Fumaric Acid
Fumaric acid’s unique combination of chemical stability, low toxicity, and versatility enables its use across a wide spectrum of industries. From food processing to industrial manufacturing and pharmaceuticals, fumaric acid plays a critical role in enhancing product performance, stability, and quality.
Food and Beverage Industry
Fumaric acid is a versatile acidulant extensively used to enhance flavor, acidity, and shelf life. NORBIDAR supplies food-grade fumaric acid tailored to meet strict safety and purity standards. Key applications include:
Baked Goods: It strengthens dough, enhances texture, and provides a pleasant sour flavor in rye, sourdough, and tortillas.
Confectionery: Used to acidify candies and jelly products, it improves shelf stability by reducing moisture absorption and retarding sucrose inversion.
Beverages: It offers more sourness per unit weight than many other acids, stabilizes pH in fruit juices and carbonated drinks, and prevents secondary fermentation in wines.
Jams and Jellies: It improves gel strength, allowing reduction in gelatin content and extending shelf life.
Feed Efficiency and Animal Nutrition
NORBIDAR provides feed-grade fumaric acid with strict control of heavy metal limits, ensuring safe and effective use. Fumaric acid enhances feed efficiency and gut health in various animals:
Poultry Feeds: In poultry feeds, it enhances nutrient absorption and aids in controlling harmful pathogenic bacteria.
Swine Feeds: For swine feeds, fumaric acid supports better weight gain and improves feed conversion rates after weaning.
Ruminant Feeds: In ruminant feeds, it helps to regulate rumen pH and maintain a healthy microbial balance.
Aquafeeds: In aquafeeds, this acid improves feed stability and boosts nutrient uptake for aquatic animals, contributing to overall health and growth.
Pharmaceuticals and Healthcare
In healthcare, fumaric acid is an active ingredient in antifungal treatments and is used in nutritional supplements to support energy metabolism. Its derivatives, particularly fumaric acid esters, are important in treating multiple sclerosis. NORBIDAR ensures pharmaceutical-grade purity and compliance with international standards for medical applications.
Antifungal Treatments: Certain fumarate derivatives exhibit antifungal activity and are incorporated into topical formulations for skin infections.
Nutritional Supplements: Iron fumarate is a common iron supplement used to treat and prevent iron-deficiency anemia.
Multiple Sclerosis Treatments: Fumaric acid esters have immunomodulatory effects and are approved for treating certain neurological diseases.
Cosmetic and Personal Care Products
Fumaric acid’s role as a pH adjuster and stabilizer extends to the cosmetic and personal care industry. It helps maintain the acidity levels in products to ensure optimal performance and safety. Key applications include:
Skincare Products: Fumaric acid as an antioxidant is used in creams, lotions, and serums to maintain a slightly acidic pH, which supports the skin’s natural barrier function.
Hair Care: In shampoos, conditioners, and hair treatments, fumaric acid helps to balance pH levels, which can enhance the effectiveness of active ingredients and improve hair texture.
Toothpaste and Oral Care: Fumaric acid is sometimes included in toothpaste formulations to maintain pH balance and improve product stability, helping to protect teeth from bacterial growth and plaque buildup.
Industrial Unsaturated Polyester Resins
Industrial applications are another major area where fumaric acid excels. It is a critical raw material for unsaturated polyester resins used in construction, automotive, and marine industries. It contributes to the production of paints, coatings, adhesives, and sealants with strong performance and durability. NORBIDAR provides consistent industrial-grade fumaric acid for reliable manufacturing processes.
Construction Materials: UPR-based materials produce building panels, roofing, and pipes, benefiting from fumaric acid’s chemical resistance and strength.
Automotive Parts: Fiberglass plastics with fumaric acid resins are lightweight, durable for car bodies and interiors.
Paints and Coatings: Fumaric acid forms alkyd resins for paints offering adhesion, gloss, and weather resistance.
Adhesives and Sealants: It creates strong, heat- and chemical-resistant cross-linked polymers.
Fumaric Acid Derivative
Fumaric acid is a dicarboxylic acid with a trans configuration, and its derivatives include salts, esters, and polymerized forms that retain the core fumaric acid structure but have different functional groups or modifications for various applications.
Fumaric acid derivatives often exhibit enhanced or distinct biological or material properties compared to fumaric acid itself. These derivatives have tailored properties that expand fumaric acid’s use in pharmaceuticals, materials science, and biomedical applications.
Fumaric Acid Esters
Esters such as dimethyl fumarate and monomethyl fumarate possess anti-inflammatory and immunomodulatory properties. They are widely used in dermatology and pharmaceutical formulations to treat conditions like psoriasis and multiple sclerosis.
Fumaric Acid Diamides
Fumaric acid diamides, such as N, N’-dimethylfumaramide, and N, N’-diphenylfumaramide, are derivatives studied for their potential as organic catalysts and ligands in metal coordination chemistry.
Fumaric Acid Salts
Fumaric acid can form salts by reacting with various bases. For example, sodium and potassium fumarate are commonly used in the food industry as pH regulators, acidulants, and flavor enhancers, providing enhanced stability and bioavailability.
Invest in High-quality Fumaric Acid


