Fumaric acid food additive is special in food because it is very sour, helps keep things stable, and saves money. Citric acid gives a taste people know well. Malic acid provides a more even flavor. Choosing the right acid changes how food tastes, how long it lasts, and its texture. In grape juice, using the fumaric acid food additive instead of citric acid can help it last longer and maintain its bright color. New studies show that the amount of acid and how you store food affect its taste, smell, and feel. The fumaric acid food additive keeps the pH steady, prevents germs from growing, and preserves grape juice quality at every step.
Overview of Fumaric, Citric, and Malic Acids
Fumaric Acid Food Additive
Fumaric acid has four carbon atoms. Scientists also call it trans-butenedioic acid. Its formula is HO2CCH=CHCO2H. Fumaric acid is found in plants like fumitory. It is also in mushrooms such as bolete. Lichen and Iceland moss have fumaric acid too. This acid helps living things turn food into energy. Grapes have a little fumaric acid. It makes grapes taste tart.
Food makers use the fumaric acid food additive for its strong sour taste. It helps keep foods fresh. The additive works well in grape juice, jams, and jellies. It keeps the pH steady. It stops germs from growing. Fumaric acid food additive is approved in the European Union as E297. It is safe for baked goods, drinks, and grape-based foods. Producers pick fumaric acid because it saves money. It also helps keep color and flavor in grape foods.
Tip: Fumaric acid food additive is great for grape products. It helps keep their color bright and taste fresh.
Citric Acid: Key Features
Citric acid is found in many fruits. Citrus fruits like lemons and oranges have a lot of it. Grapes have some citric acid too, but less. Citric acid gives grape juice a tangy taste. It helps cells get energy.
Manufacturers use citric acid to control acidity. It helps keep foods fresh. Citric acid stops spoilage in grape juice and drinks. It is approved as E330 in the European Union. Citric acid is used in canned foods, snacks, ice creams, and soft drinks. In grape products, citric acid keeps the taste bright. It also helps keep the color stable.
| Acid Name | E-number | Function(s) | Regulatory Status / Significance in Food Regulation |
|---|---|---|---|
| Citric acid | E330 | Acidity regulator, preservative | Approved in the EU; widely used in canned foods, snacks, ice creams, soft drinks |
Malic Acid: Features and Uses
Malic acid is an organic acid. Its formula is HO2CCH(OH)CH2CO2H. It is found in many fruits, including grapes. Malic acid gives grapes their sour taste. It helps balance sweetness. It also helps cells make energy.
Food producers use malic acid to improve flavor. It helps control acidity. Malic acid is approved as E296 in the European Union. It works as an acidity regulator, flavoring agent, and color stabilizer. In grape juice and grape candies, malic acid makes a smooth, lasting sourness.
| Acid Name | Chemical Definition | Natural Sources | Additional Notes |
|---|---|---|---|
| Fumaric acid | trans-butenedioic acid; formula HO2CCH=CHCO2H; a four-carbon dicarboxylic acid | Found in fumitory, bolete mushrooms, lichen, Iceland moss, grape | Intermediate in the citric acid cycle; fruit-like taste; used as food additive (E297) |
| Malic acid | Organic dicarboxylic acid; formula HO2CCH(OH)CH2CO2H; naturally as L-isomer | Occurs naturally in fruits, including grape | Intermediate in the citric acid cycle; used as food additive |
| Citric acid | N/A | Citrus fruits, grape | Used as food additive (E330); acidity regulator, preservative |
Fumaric vs. Citric and Malic Acids: Key Differences
Sourness & Acidifying Power
Fumaric acid, citric acid, and malic acid all change how grape foods and wines taste. How sour they are depends on their structure. Fumaric acid and malic acid have two carboxyl groups. Citric acid has three carboxyl groups. At the same amount and pH, fumaric acid and malic acid taste more sour than citric acid. This means grape juice and wine taste sharper with fumaric acid or malic acid. The way acids work with taste buds also matters. Fumaric acid tastes strong and tart. This is good for acidified wines and grape drinks. Citric acid gives a quick, bright sourness. Malic acid gives a smoother, longer tartness. These acids change how grape foods and wines taste.
Solubility
Solubility tells us how well acids mix in grape foods and wines. Fumaric acid does not dissolve well in water. It only dissolves about 5.3 grams per liter at room temperature. Citric acid and malic acid dissolve much better. Their solubility is about 960 grams per liter and 845 grams per liter. In wine and grape must, fumaric acid dissolves a little more but still less than the others. This means food makers must watch how fumaric acid mixes in grape juice and wine. They often use heat to help it dissolve. The chart below shows how well each acid dissolves in water and wine:
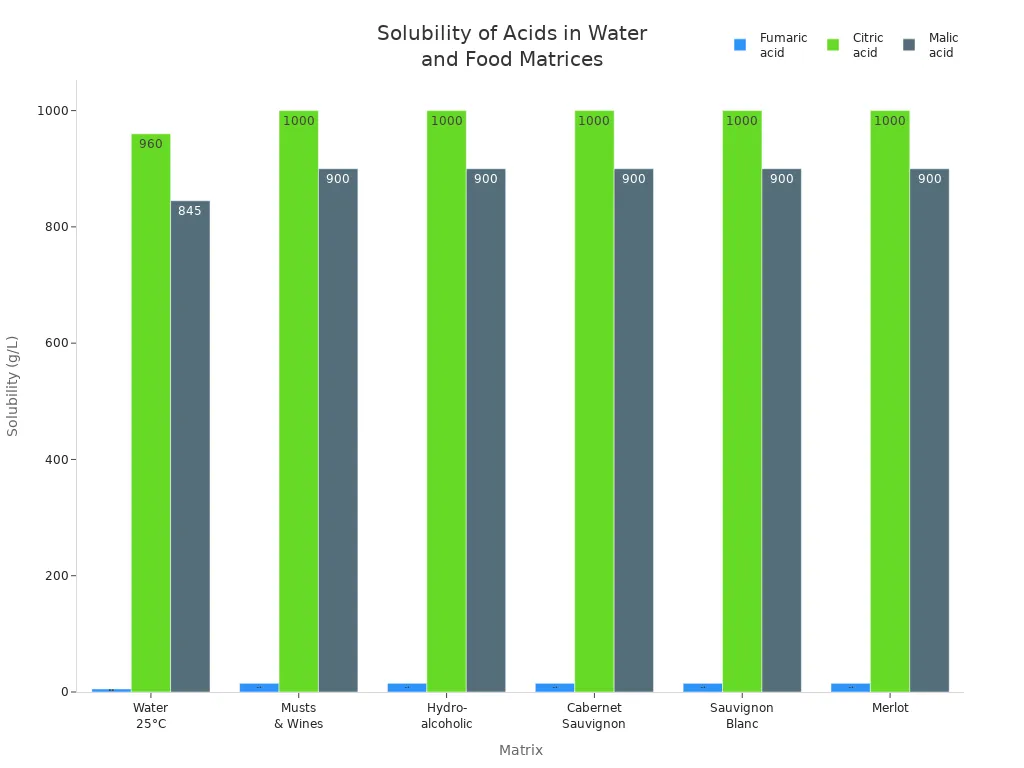
Low solubility can change the taste and sourness in grape foods. Citric acid and malic acid dissolve easily, so they work well in grape drinks and wines. Fumaric acid needs special care to make sure the taste is even.
Buffering Capacity
Buffering capacity shows how well an acid keeps pH steady in grape foods and wines. Malic acid has a higher buffering index than citric acid. This means it keeps pH steady better when making grape foods. This helps the texture and stability of grape jellies and wines. Fumaric acid also lowers pH, but its buffering is not studied as much in food. All three acids help keep acidified wines fresh and safe by keeping pH low. Malic acid’s strong buffering helps make grape gels firmer. Citric acid helps control acidity but does not buffer as well as malic acid. Fumaric acid’s buffering is mostly known in animal feed, but it still helps acidify grape foods and wines.
Sensory Impact
The way grape foods and wines taste depends on which acid is used. Citric acid gives a sharp, bright taste that goes away fast. Malic acid gives a smooth, lasting tartness and balances sweetness. Fumaric acid makes a strong tartness and can make your mouth feel dry. This makes fumaric acid taste different from the others. In acidified wines, fumaric acid brings out fruit flavors and gives a special taste. These acids change the taste, mouthfeel, and smell of grape foods and wines. Studies show citric acid is used for its bright taste, malic acid for smooth tartness, and fumaric acid for strong, drying effects. The acid you pick changes the taste and feel of grape foods and wines.
Note: How acids change taste and feel is important for grape foods and wines. Fumaric acid, citric acid, and malic acid each give special effects that help make the best taste.
Applications of Fumaric, Citric, and Malic Acids Across Food
Beverage and Flavor Applications
Drink makers use fumaric acid, citric acid, and malic acid to change how drinks taste. Citric acid is used the most in drinks like sports drinks, sodas, energy drinks, and fruit juices. It gives grape drinks a sharp, fresh sourness that makes them taste and smell better. Malic acid gives a smooth, long-lasting tartness and helps balance sweetness. This is why it is used in grape juices and flavored waters. Fumaric acid is not used as much, but it gives a strong, lasting sourness and helps make grape drinks more acidic. Because fumaric acid does not dissolve well, companies use less of it. Even a small amount can change taste and help drinks last longer.
| Acid Type | Common Food & Beverage Categories | Usage / Replacement Rates / Notes |
|---|---|---|
| Fumaric Acid | Bakery (rye, sourdough breads, English muffins), fruit juice drinks, wine, confectioneries, jellies & jams, alginate desserts, gelatin desserts, pie fillings, egg white foams, animal feed | – Replaces citric acid at 0.91 g fumaric acid to 1.36 g citric acid for same taste – In jams/jellies, 2 lbs fumaric acid replaces 3 lbs citric/malic/tartaric acid – In gelatin desserts, 1 lb citric acid replaced by 0.6-0.7 lb fumaric acid – Provides stronger sourness per unit weight, reducing acidulant cost – Non-hygroscopic, improves product stability and shelf life |
| Citric Acid | Beverages: sports drinks, soft drinks, energy drinks, fruit juices, alcoholic beverages | Widely used as acidulant; specific usage rates not detailed in sources but noted for flavor enhancement and pH control |
| Malic Acid | Beverages: sports drinks, soft drinks, energy drinks, fruit juices, confectionery (similar to fumaric acid) | Used as acidulant and sourness agent; specific usage rates not provided but recognized for sourness contribution |
Citric acid and malic acid are the main acids in drinks. They make drinks taste better, keep them stable, and help them last longer. Fumaric acid is strong and stops germs from growing, so it helps keep grape drinks safe. It is used in grape juices and wines to give a steady, tart taste and help them last longer.
Bakery Applications
Bakers use fumaric acid, citric acid, and malic acid to control how sour dough is, make bread softer, and help bread last longer. Fumaric acid makes bread bigger, especially rye and sourdough, by helping break down proteins and starch. It also works as an acid and antioxidant, which slows down bread going stale and stops germs. Citric acid makes bread softer and helps yeast work better. Malic acid also helps with texture and size, but not as much as citric acid.
| Functional Role / Performance Aspect | Fumaric Acid (FA) | Citric Acid (CA) | Malic Acid (MA) |
|---|---|---|---|
| Effect on Specific Volume (SV) | Increased SV to 6.071 mL/g; strong biochemical impact on protein and starch hydrolysis | Highest increase in SV to 6.232 mL/g; noted for crumb softening effects | Increased SV to 6.034 mL/g; improves texture and volume but less than CA |
| Impact on Moisture Content | Decreases moisture content | Decreases moisture content | Decreases moisture content |
| pH Effect | Lowers pH, contributing to acidification | Lowers pH, contributing to acidification | Lowers pH, contributing to acidification |
| Yeast Activity | Enhances yeast activity | Enhances yeast activity | Enhances yeast activity |
| Gas Retention in Dough | Reduces gas retention capability | Reduces gas retention capability | Reduces gas retention capability |
| Proteolysis and Amylolysis | Most significant increase in proteolysis and amylolysis; reduces molecular weight of proteins and starches | Moderate proteolysis; contributes to crumb softening | Moderate proteolysis; improves texture and volume |
| Texture and Crumb Softness | Improves texture via biochemical effects | Noted specifically for crumb softening | Improves texture but less than CA |
| Shelf Life and Antimicrobial Role | Acts as acidifying and antioxidizing agent; delays starch retrogradation and increases antimicrobial activity | Same acidifying and antioxidizing roles | Same acidifying and antioxidizing roles |
Fumaric acid helps stop bread and pastries from spoiling and makes them last longer. It does not take in water from the air, so dry mixes stay dry and fresh. This makes it good for keeping bakery foods safe.
Dairy Applications
In dairy foods, fumaric acid, citric acid, and malic acid help with sourness, taste, and texture. Citric acid gives a sharp, lemon-like taste and lowers pH to stop germs. This helps keep dairy foods safe. Malic acid gives a smooth, fruity taste and helps balance flavors in grape yogurts and cheeses. Fumaric acid controls pH, helps proteins form gels, and makes grape-flavored dairy foods taste better.
| Acidulant | Role in Dairy Applications |
|---|---|
| Citric acid | Provides sharp, citrusy flavor; enhances flavor; lowers pH to inhibit microbial growth, aiding preservation. |
| Malic acid | Offers smooth, fruity taste; modifies texture; helps balance flavor and moderately preserves by lowering pH. |
| Fumaric acid | Improves texture through pH regulation affecting protein gelation; enhances flavor; extends shelf life due to antimicrobial properties and heat stability. |
Adding fumaric acid to cheese and yogurt helps them last longer by stopping germs. It can handle heat, so it works well in processed dairy foods and keeps them safe.
Confectionery Applications
Candy makers use fumaric acid, citric acid, and malic acid to make grape candies, jellies, and desserts taste special. Fumaric acid is about 1.5 times stronger than citric acid, so you need less to get the same sour taste. It does not soak up water, so powdered foods like gelatin desserts and pie fillings last longer. Citric acid gives a quick, sharp tartness and stops browning. Malic acid gives a smooth, long tartness and makes fruit flavors stronger.
| Acid | Comparative Advantages in Confectionery Applications |
|---|---|
| Fumaric Acid | – Approximately 1.5 times stronger acidity than citric acid. – Very low moisture absorption, extending shelf life in powdered products like gelatin desserts and pie fillings. – Used in smaller quantities to achieve similar taste effects. – Helps prevent crystal formation in refrigerated doughs. – Does not chelate metals like copper or iron. |
| Citric Acid | – Most widely used acidulant in the food industry (over 60% of acidulants consumed). – Highly soluble in water. – Provides a sharp ‘burst’ of tartness. – Strong metal chelation properties, preventing haze and browning. – Wide buffer range (pH 2.5–6.5). – Used in hard confectionery to impart tartness and prevent sucrose inversion and browning. – Acts as a preservative and pH controller in beverages and desserts. |
| Malic Acid | – Imparts a smooth, lingering tart taste that masks aftertastes of low-caloric sweeteners. – Has taste-blending and flavor-fixative properties. – Relatively low melting point allows homogeneous distribution in food systems. – Enhances fruit flavors, especially strawberry and cherry. – More hygroscopic; encapsulated forms preferred in dry mixes to prevent lumping and browning. – Used in sour confectionery and synergizes with aspartame to reduce sweetener levels. |
| Combined Use | – Cogranulation of citric acid with malic and fumaric acids creates new tart flavor profiles, indicating complementary applications in confectionery. |
Fumaric acid helps stop germs and spoilage in grape jellies and candies. This keeps them safe and helps them last longer.
Processed Foods
Processed foods use fumaric acid, citric acid, and malic acid to make foods more sour, keep them safe, and improve taste. Citric acid is used a lot to add sourness, make flavors better, and stop browning in grape foods. Malic acid makes vegetables, canned fruits, and jellies taste more tart. Fumaric acid is found in fruit drinks, breads, pie fillings, wine, poultry, jelly, and jello. All three acids help control acidity and stop germs from growing.
Fumaric acid is special because it is natural and breaks down easily. It helps foods last longer by lowering pH, which stops bacteria, mold, and yeast. Its germ-fighting power also stops spoilage. Fumaric acid stops browning and acts as an antioxidant, protecting grape foods from damage. All three acids are safe if used right, and food labels must show them, often with their E-numbers.
Wines and Wine
Fumaric acid is important in wines and grape musts. It makes wine more acidic, lowers pH, and gives a strong, lasting sourness. Fumaric acid stops malolactic fermentation by slowing or stopping lactic acid bacteria. If you use 300 mg/L or more, it can delay fermentation for over 50 days. This keeps malic acid in the wine and helps it stay fresh and sour. This works better than sulfur dioxide and keeps wine at the right acidity.
Fumaric acid is as strong or a bit stronger than citric acid, so it lowers pH and makes wine more stable. Citric acid can let unwanted germs grow and cause acetic acid, but fumaric acid stops this and keeps wine safe. Using fumaric acid means less sulfur dioxide is needed, which helps keep wine color and grape color strong.
Fumaric acid does not dissolve as well as citric or malic acid, so winemakers must mix it carefully. Its strong sourness and germ-fighting power make it great for wine and food safety. Fumaric acid is not as good as ascorbic acid or glutathione at stopping color loss, but it is best at stopping fermentation and keeping wine sour. Ascorbic acid and glutathione help keep wine color and smell, but fumaric acid is used for stopping germs and making wine more acidic.
Note: Fumaric acid helps wine by stopping germs, keeping it sour, and making it taste better. Using it in wines helps keep them safe, stops spoilage, and keeps grape wines tasting fresh.
Safety of Fumaric, Citric, and Malic Acids in Food
Toxicity and Health Implications of Fumaric, Citric, and Malic Acids in Food
Fumaric acid, citric acid, and malic acid are found in many foods and drinks. They are also used in wines. Scientists and food experts have checked if these acids are safe. Fumaric acid is not toxic. The FDA and EFSA say it is safe. The FDA says people can have up to 10 mg for each kilogram they weigh every day. Citric acid and malic acid do not have strict daily limits. Experts agree they are safe in the amounts used in food and wine. The table below shows the daily intake values:
| Acid | Established ADI (mg/kg bw/day) | Regulatory Authority / Notes |
|---|---|---|
| Fumaric Acid | 10 | FDA and EFSA both set ADI at 10 mg/kg body weight per day; FDA designates it as GRAS; EFSA approves use with no specific limits. |
| Malic Acid | No specified ADI for dl-malate; conditional ADI of 100 mg/kg bw/day for d-isomer withdrawn | JECFA initially set conditional ADI for d-malic acid due to limited data, later withdrawn after further data; no safety concern at current intake levels for L-malic acid; restrictions remain for infants. |
| Citric Acid | No explicit ADI stated; exposure estimates related to calcium citrate malate use | EFSA AFC Panel concluded no safety concern at estimated exposure levels (~1400 mg/day citric acid equivalent); no formal ADI established. |
Fumaric acid helps keep wine and grape foods safe by stopping bad germs. Citric acid and malic acid also help fight germs in wine and other foods. Studies show these acids do not hurt people when used in normal amounts. Malic acid can bother skin or eyes if used in large amounts, but this does not happen in food or wine.
Allergenicity of Fumaric, Citric, and Malic Acids
Most people can eat foods and drink wines with fumaric acid, citric acid, or malic acid without problems. A few people may get mild reactions from fumaric acid, like headaches, diarrhea, or feeling sick. These reactions are rare. Citric acid and malic acid almost never cause allergies in wine or food. Sometimes, people with sensitive skin may feel a little irritation, but this is not common in wine or grape foods.
Note: If someone feels sick after eating foods or drinking wines with these acids, they should talk to a doctor.
Regulatory Approvals and Global Standards for Fumaric, Citric, and Malic Acids in Food
Food safety rules help keep people safe when they eat wine and grape foods. The European Union says fumaric acid (E297), citric acid (E330), and malic acid (E296) are okay to use in food. These acids must follow strict rules for quality and labels. The FDA in the United States lets food makers use fumaric acid in foods and wines up to 0.1% by weight. The EU and Asia also have rules, but only the US gives a clear limit for fumaric acid. Citric acid and malic acid do not have strict limits, but food makers must follow safety rules. All three acids are made using safe methods with special microorganisms. These rules make sure wines and grape foods stay safe for everyone.
| Region | Regulatory Limits for Fumaric Acid | Notes on Citric and Malic Acid Regulatory Limits | Market and Regulatory Environment Summary |
|---|---|---|---|
| US | FDA limits fumaric acid use in foods to 0.1% by weight | No specific regulatory limit data available | FDA closely monitors fumaric acid; market driven by food and beverage industry |
| EU | Stringent regulations favoring food safety and quality; exact limits not detailed | No specific regulatory limit data available | EFSA monitors fumaric acid; regulations are strict, supporting food preservation and pH adjustment uses |
| Asia (APAC) | No specific regulatory limits detailed; relatively lax regulatory environment | No specific regulatory limit data available | Large market share (40.5%), driven by demand in food, cosmetics, pharmaceuticals; regulatory environment supports production growth |
Tip: Always look for E-numbers like E297, E330, or E296 on food labels when picking wines or grape foods.
Food experts pick fumaric acid, citric acid, or malic acid for different reasons. They look at how long food lasts, how much it costs, and how it tastes. Fumaric acid is good for dry mixes, candies, and tortillas. It does not take in much water and gives a strong sour taste. Citric acid is used most in drinks and processed foods. It quickly controls pH and tastes good to many people. Malic acid is often mixed with citric acid in juices. It helps make the taste better. One study showed mixing 90% citric acid with 10% malic acid made orange juice taste better. Market reports say citric acid is the cheapest and most used.
| Application | Fumaric Acid | Citric Acid | Malic Acid |
|---|---|---|---|
| Dry mixes, candies | Best for shelf life | Less effective | Not preferred |
| Beverages, juices | Strong sourness, less soluble | Most used, quick pH control | Enhances sensory, used with citric acid |
Food makers and buyers should pick the acid that fits their needs, follows rules, and gives the taste they want.
FAQ
What makes fumaric acid different from citric and malic acids in food?
Fumaric acid gives a stronger sour taste than citric or malic acids. It does not absorb water easily. Food makers use less of it to get the same sourness. It also helps foods last longer.
Can people with allergies eat foods with these acids?
Most people can eat foods with fumaric, citric, or malic acids safely. Allergic reactions are rare. Some people may feel mild discomfort. If someone feels sick, they should talk to a doctor.
Why do food companies use fumaric acid in dry mixes?
Fumaric acid does not take in moisture from the air. Dry mixes stay fresh longer. It also gives a strong sour taste. This helps keep powdered foods like cake mixes and candies safe and tasty.
Is fumaric acid safe for children?
Yes, fumaric acid is safe for children when used in normal food amounts. Food safety groups have checked it. They set limits to keep foods safe for everyone, including kids.
Which acid works best for making drinks taste sour?
Citric acid works best for a quick, sharp sour taste in drinks. Malic acid gives a smooth, lasting tartness. Fumaric acid gives a strong sourness but does not dissolve as well in drinks.